AP Chemistry Lab Report Rubric
advertisement

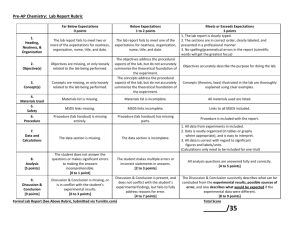
AP Chemistry: Lab Report Rubric Far Below Expectations 0 points Below Expectations 1 to 2 points 1. Heading, Neatness, & Organization The lab report fails to meet two or more of the expectations for neatness, organization, name, title, and date. The lab report fails to meet one of the expectations for neatness, organization, name, title, and date. 2. Objective(s) Objectives are missing, or only loosely related to the lab being performed. 3. Concept(s) Concepts are missing, or only loosely related to the lab being performed. 4. Procedure Procedure (lab handout) is missing entirely. 5. Experimental Data The objectives address the procedural aspects of the lab, but do not accurately summarize the theoretical foundation of the experiment. The concepts address the procedural aspects of the lab, but do not accurately summarize the theoretical foundation of the experiment. Procedure (lab handout) has missing parts. Meets or Exceeds Expectations 3 points 1. The lab report is clearly written (or typed). 2. The sections are in correct order, clearly labeled, and presented in a professional manner 3. No spelling/grammatical errors in the report of scientific words Objectives accurately describe the purpose for doing the lab. Concepts (theories, laws) illustrated in the lab are thoroughly explained using clear examples. Procedure is included with the report. 1. All data from experiments in included 2. Data is neatly organized (in tables or graphs where appropriate), and is easy to interpret. 3. All data is correct with regard to significant figures and labels/units. The data section is missing. The data section is incomplete. 6. Analysis [5 points] The student does not answer the questions or makes significant errors to making the answers incomprehensible. [0 to 1 point] The student makes multiple errors or incorrect statements in answers. [2 to 3 points] All analysis questions are answered fully and correctly. [4 to 5 points] 7. Discussion & Conclusion [10 points] Discussion & Conclusion is missing, or is in conflict with the student’s experimental results. [0 to 3 points] Discussion & Conclusion is present, and does not conflict with the student’s experimental findings, but fails to fully address reasons for error. [4 to 7 points] The Discussion & Conclusion succinctly describes what can be concluded from the experimental results, possible sources of error, and also describes what would be expected if the experimental data were different. [8 to 10 points] Examples: The following table gives examples of laboratory answers for a “Density of Salt Solutions” lab. Below Expectations Meets or Exceeds Expectations 1 to 2 points 3 points “Determination of the Relationship Between the 1. “Salt Solution Density Lab” Density and Concentration of Sodium Chloride Solutions” Heading, Ms. Regan Neatness, & August 17th, 2011 Organization “The purpose of the lab is to develop a mathematical model relating the “The purpose of the lab is to learn to find the density concentration of a solution to its density, and to use this model to determine the 2. of salt solutions.” concentration of solutions of unknown concentration from their densities.” Objective(s) “Density is defined as mass divided by volume.” 3. Concept(s) “Density tells us how much matter is in a given space. Likewise, concentration tells us how much matter is present in a certain amount of another substance, such as how many moles of solute are in 1 liter of solution (molarity) or how many grams of solute are in 100 grams of solution (mass %). Because density and concentration both measure how compact a sample of matter is, we expect to see a direct relationship between them. Concentration of NaCl solution Mass of solution (g) 5. Experimental Data 5% solution = 10.012 g 10% solution = 10.180 g 15% solution = 10.230 g 6. Analysis Density = 10.012 g/10.00 mL = 1.0012 g/mL (significant figures error) Density = 10.012/10.00 = 1.001 g/mL (units not present in calculation) Density = m/V (fundamental equation shown) Density = 10.012 g/10.00 mL = 1.001 g/mL (units present throughout calculation, significant figures rules observed) “We demonstrated that it is possible to measure the densities of solutions, and to find the concentrations of unknowns.” “We demonstrated that a linear relationship exists between the density and concentration of sodium chloride solutions, and that the relationship can be used to make predictions about the properties of solutions of unknown concentration.” “We showed that as the concentration of a solution increases, the density of the solution also increases linearly. Our data supports this conclusion. The purpose of the lab was fulfilled.” “As the concentration of a solution increases, the density of the solution increases in linear fashion. Our data supports this concept. The purpose of the lab was fulfilled when we were able to use the mathematical model for this linear relationship to predict the concentration of solutions of unknown concentration based on their densities.” “However, we failed to take into account the mass of the graduated cylinder when finding the mass of the solutions.” “However, we failed to take into account the mass of the graduated cylinder when finding the mass of the solutions. As a result, the mass of each solution was too high, and the resulting density was also too large.” 7. Discussion & Conclusion 5% 10% 15% 10.012 10.180 10.230