a case of hatcliffe extension, harare
advertisement

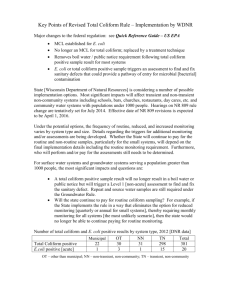
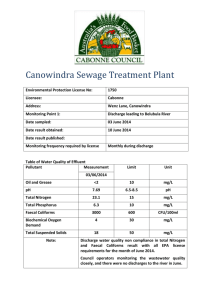
1 Effectiveness of Solar Disinfection (SODIS) as a Purification method for Bacteriologically Contaminated Drinking Water in Zimbabwe: A case of Hatcliffe Extension, Harare Dorika Madzingamiri, Maxwell Barson, Sibonginkosi Maposa *Institute of Water and Sanitation Development, Alexandra Park, Harare, Zimbabwe. *Department of Biological Sciences, University of Zimbabwe, Mt Pleasant, Harare, Zimbabwe. Email: barson001@yahoo.co.zw / barson@science.uz.ac.zw. *Water and Sanitation for Development Group, Council for Scientific and Industrial Research, P O Box 395, Pretoria 0001, South Africa. Email BMaposa@csir.co.za/ bongi.maposa@gmail.com *Corresponding author email: dorika@iwsd.co.zw ABSTRACT The study tested the effectiveness of solar disinfection (SODIS) against coliform bacteria, and specifically Escherichia coli in drinking water from household wells in the peri-urban settlement of Hatcliffe Extension near Harare, Zimbabwe. The samples were collected from the drinking water wells of 13 households over a twomonth period (April – May 2008) into clear 2-litre PET bottles. The samples were exposed to sunlight for six hours, with hourly examination to determine the rate of bacterial inactivation. The number of coliforms was estimated using the most probable number (MPN) method and presence/absence analysis was done for E.coli. The untreated water from all the wells was found to have very high levels of faecal coliforms, ranging from 625 coliforms /100ml to above 1100 coliforms /100ml. The number (MPN) of total coliforms decreased considerably between the 4/5-hour exposure periods in 61.5% of the water samples, suggesting that it could be the minimum time required for bacterial inactivation under field conditions. The rate of inactivation of the coliforms followed an exponential curve increasing with duration of exposure, ranging from 30.5% after the first hour to 74.1% in the last hour. At the end of the six hours of exposure, all of the samples had zero coliform counts except for one from a site with particularly high turbidity. There also appeared to be a pH influence on the rate of coliform inactivation with the highest rate of inactivation appearing to be at pH 7.7. Out of the 13 water samples examined, 92.3% showed total absence of E. coli after six hours, with the greatest decline (74.1%) occurring in the last hour of exposure. After 5 hours of exposure, only 38% of the samples showed absence of E.coli suggesting that six hours was the critical exposure period for E.coli. The possibility of regrowth of the inactivated coliforms was also tested and although no definite conclusions could be drawn from the data in this study, the findings indicate that the phenomenon of re-growth in field applications merits further investigation. These findings suggest that SODIS can be applied as a household water treatment method to effectively inactivate coliform bacteria, notably E. coli but pH and turbidity of the water as well as the exposure period need careful optimization in field application as they appear to have potential to impact on the efficacy of the process. 2 Introduction The access to safe drinking water is recognized as a key determinant of the infectious disease burden especially in developing countries yet 1.1 billion people, 20% of the world’s population are without it (WHO, 2008). Whilst indeed obtaining clean drinking water is a primary concern for people living in the developing world (Joyce et al., 1995), urban and peri-urban communities in these countries are emerging as a particular challenge in this regard. In developing countries the rate of urbanization has accelerated with large numbers of poor population groups pouring into the informal suburbs of major cities, often exacerbating already existing acute water supply problems (Collignon et al., 1999). This is in a context where water supplies developments in urban areas are typically lump sum investments as opposed to gradual or incremental. For example, a small increase in the size of a pipeline requires a whole new pipeline, which needs to be purchased (Cairn cross and Feachem, 1993). Thus the newly established settlements are often poorly supplied with safe drinking water. Furthermore, large proportions of the inhabitants in these underprivileged areas cannot afford the costs of individual connections to their homes. Consequently, they often turn to alternative systems that they can more easily access (Collignon et al, 1999), although most of these alternatives are not safe. Household based water treatment and safe storage of drinking water (HWTS) technologies are now recognized as cost effective measures for the provision of safe drinking water. The accumulation of evidence on the post collection contamination of stored household water (Wright et al., 2004) has provided impetus for the development of these household based interventions. HWTS is effective, simple, and inexpensive (WHO, 2003). There are several methods of purifying drinking water after it has been carried to the home and these may be classed as physical or chemical technologies. The chemical water treatment processes rely on disinfectants to inactivate or kill waterborne micro-organisms. These disinfectants include the simple household bleach or chlorine tablets (Zwane and Kremer, 2007). The physical methods include boiling, filtration and solar disinfection. Boiling is the oldest known method of treating drinking water at household level (Sobsey, 2002). Its effectiveness in producing water of acceptable microbiological quality is accepted. Filtration relies on the physical removal of pathogens from water by a filter medium. It has also been used with varying effectiveness since ancient times (Clasen and Cairn cross, 2004). The effectiveness of filtration in removing microbes varies widely, depending on the type of microbe as well as the type and quality of the filtration medium or system. This study focused on the application of solar disinfection in non-outbreak conditions in a developing country in a peri-urban setting. This use of the sun (solar energy) to disinfect drinking water is a technology that is presently undergoing rapid development. It has been shown to reduce the bacterial content of water and thus offers a method for disinfection of drinking water that requires few resources and no expertise. The technology dates back a few centuries when it was practiced in India and it has been recognized in modern times since studies by Acra et al (1984). Solar disinfection relies on the synergistic effect of Ultra Violet (UV) rays (wavelength 320-400nm) and heat to destroy the pathogens in drinking water (Sobsey, 2002). UV –A reacts with oxygen dissolved in the water to produce highly reactive forms of oxygen that damage the DNA of bacteria thus killing them. The efficacy of SODIS in eliminating a wide range of pathogens is documented. It is known to be effective against Escherichia coli, Vibrio cholerae, Salmonella typhimurium Shigella dysenteriae Type I, Pseudomonas aeruginosa, Candida albicans, Fusarium solani, and the trophozoite stage of Acanthamoeba polyphaga (Joyce et al, 1996; Kehoe et al, 2004; Lonnen et al, 2005 and Smith et al, 2000). However, there is still limited evidence on the virucidal performance of SODIS (EAWAG, 2007), although it is expected to be effective at the conditions normally achieved during the process. The rate of decay of pathogens in real life conditions is not known and this can reasonably be expected to limit its field application, thus the need for its further investigation in this project. This study aimed to determine the potential application of solar disinfection as a household based water purification method in the peri-urban settlement of Hatcliffe Extension. Specifically it sought to achieve the following: 1. Determination of the rate of destruction of coliforms in water drawn from the unprotected wells in Hatcliffe Extension under field conditions; 3 2. Determination of the time required, under field conditions, to completely disinfect faecally contaminated water from the unprotected wells; and 3. Investigation of the possibility of reactivation of coliform bacteria in drinking water after six hours of exposure to sunlight. Study area Hatcliffe Extension is a peri-urban settlement located 21km north of Harare city centre and is divided into four administrative sections. It is one of the areas most affected by the government’s clean-up campaign, Operation Murambatsvina, a programme of mass forced evictions and demolition of homes and informal businesses in 2005. The settlement has been relatively unstable since its re-establishment in 2002. Presently, the area has approximately 2197 households, which are temporarily-made tents and was selected for this particular study based on its reliance on untreated water for drinking and the residents’ practice of storing water in the home. Although some residents have standpipes on their residential plots, this municipal supply is rarely available and when it is, the water is muddy and contains algae-like deposits (Spectrum ITS, 2008). Residents thus rely on water from unprotected wells they have on their residential plots. The wells, though covered at the surface, are not protected underground such that the water is prone to contamination from nearby pit latrines. In addition, some households do not have their own wells, so they store water in plastic or metal containers and the water is thus prone to contamination. 4 Experimental Water samples were taken once every week between April and May 2008 from 13 randomly selected households in Hatcliffe Extension for microbiological analyses. Six samples were collected from each household well using the drawing methods that are normally used by the household. Each household well was sampled once. At each household well, six 2 litre PET (polyethylene terephthalate) bottles and an additional one collected into a sterile bottle were obtained. Each bottle was filled up to ¾, shaken for approximately 20 seconds and then completely filled to allow oxygen build-up in the container since this gas aids the process of solar disinfection. Conductivity, dissolved oxygen, air temperature, water temperature and pH were measured at the site. pH, Dissolved Oxygen (DO) and temperature were measured using the HACH LDOT meter (model QH20). Conductivity was measured with a conductivity meter (WTW-model LF330) and turbidity was measured spectrophotometrically. The 2 litre PET bottles were exposed to sunlight for six hours on roof-top. For each sample set (household), the enumeration of total coliforms and presence of E.coli before exposure were determined using the sample collected into the sterile bottle. After an hour a bottle was removed for each household set and analyzed for total coliform count as well as presence/absence of E. coli. This was then done every hour up to 6 hours. The water was tested for E. coli to check for re-growth of test microorganisms subsequent to being placed for 24 hours in darkness, after the 6 hours of exposure to the sun. Presumptive and confirmatory tests for E. coli, as well as its differential isolation were performed using the MPN method according to Bailey and Scott (1982). The total coliform counts were done. Simple linear regression was used to test the hypothesis that there is a relationship between the number of coliforms (log10 - transformed MPN values) in a water sample after every hour of exposure and the duration of exposure to sunlight, both for each site (household) as well as for the combined sites. One-way ANOVA was used to test the significance of this relationship among water samples from different sites/regions (Bailey, 1995). The laboratory set-up and conditions satisfied the assumptions for parametric tests, hence the preference for these analytical methods. Minitab (Release 10.5 Xtra) was used for the analysis while Sigma Plot (Version 9.0) was used to plot the graphs. 5 Results The number of coliforms progressively decreased with an increase in exposure time. The number of total coliforms after every hour for all sites combined indicates the general trend of the relationship (Fig 1). The standard error decreased as the exposure time increased reaching zero at 5 and 6 hours. The greatest percentage decline in the total coliform count occurred between 4 and 6 hours of exposure; 72.7% between 4 and 5 hours & 74.1% between 5 and 6 hours (Table 7). Overall, a two log reduction in the total coliform count was achieved after 4 hours of exposure. TABLE 7. Rate of E. coli destruction after every one hour for the combined sites. Time Average MPN/100ml Percentage destruction after every hour 0 953 0% 1 662 291/953 x 100 = 30.5 % 2 388 274/662 x 100 = 41 4 % 3 141 247/388 x 100 = 63.7 % 4 97 44/141 x 100 = 31.2% 5 27 70/ 97 x 100 = 72.7% 6 7 20/ 27 x 100 = 74.1 % Linear regression analysis confirmed a significant negative relationship between time of exposure and the total coliforms MPN after every hour (Figs 1-14). 3.5 y=3.15-0.480x 1200 y=3.17-0.362x P=0.000 r2=80.1% 1000 Log MPN/100ml 2.5 MPN/100ml 800 600 2.0 1.5 1.0 400 0.5 200 0.0 0 0 1 2 3 4 5 6 7 0 2 4 Time (hrs) Time (hrs) Fig. 1 P=0.000 r2=93.4% 3.0 Fig. 2 6 6 3.5 y=3.29-0.407x P=0.000 2 r =92.8% 3.5 2 y=2.60-0.313x; P=0.000; r =74.9% 3.0 3.0 2.5 Log MPn/100ml Log MPN/100ml 2.5 2.0 1.5 1.0 2.0 1.5 1.0 0.5 0.5 0.0 0 1 2 3 4 5 6 7 0.0 0 Time (hrs) 1 2 3 4 5 6 7 Time (hrs) Col 1 vs Col 3 3.5 Plot 1 Regr y=3.25-0.316x P=0.002 r2=88.6% 3.0 Log MPN/100ml 2.5 2.0 1.5 1.0 0.5 0.0 0 Fig. 3 Fig. 4 3.5 y=3.30-0.381x P=0.000 2 r =94.6% 3.0 Log MPN/100ml 2.5 2.0 1.5 1.0 0.5 0.0 0 1 2 3 4 5 6 7 Time (hrs) Fig. 5 Fig. 6 1 2 3 4 Time (hrs) 5 6 7 7 3.5 y=3.23-0.363x r2=91.8% P=0.001 3.0 4 y=3.35-0.263x 2 P=0.012 r =74.6% 3 2.0 Log MPN/100ml Log MPN/100ml 2.5 1.5 1.0 0.5 2 1 0.0 0 1 2 3 4 5 6 7 0 Time (hrs) 0 1 2 3 4 5 6 7 Time (hrs) Fig. 7 Fig. 8 3.5 y=2.93-0.325x 3.0 P=0.000 2 3.5 R =96.8% y=3.07-0.381x P=0.000 r2=98.2% 3.0 2.5 2.0 Log MPN/100ml Log MPN/100ml 2.5 1.5 1.0 2.0 1.5 1.0 0.5 0.5 0.0 0 1 2 3 4 5 6 7 0.0 0 Time (hrs) 1 2 3 4 5 6 7 Time (hrs) Fig. 9 Fig. 10 3.5 3.5 P=0.000 r2=95.5% r2=96.0% 2.5 Log MPN /100ml 3.0 2.5 2.0 2.0 1.5 1.0 1.5 0.5 1.0 0.0 0.5 0 1 2 3 4 5 6 7 0 1 2 3 4 Time (hrs) Time (hrs) Fig. 11 P=0.000 3.0 y=2.90-0.352x Log MPN/100ml y=2.69-0.381x Fig. 12 5 6 7 8 3.5 3.5 y=2.87-0.396x 3.0 y=2.91-0.345x 3.0 r2=92.5% P=0.001 2.5 Log MPN /100ml 2.5 Log MPN /100ml P=0.001 r2=92.1% 2.0 1.5 2.0 1.5 1.0 1.0 0.5 0.5 0.0 0.0 0 1 2 3 4 5 6 7 0 1 Fig. 13 2 3 4 5 6 7 Time (hrs) Time (hrs) Fig. 14 The slope of each regression line is the regression coefficient of MPN values (y) on time (x), and it measures the average rate by which MPN decreases for a unit increase in time. This shows the rate at which coliforms were inactivated by the sun in this study. The data used for plotting the scatter graphs is shown in Tables 1 and 2 below. Table 3 shows the raw data from which the logarithm values were calculated. TABLE 1. Log – transformed MPN values (including error and mean values for combined sites) Time Log values Error values Mean values 0 2.9791 55.3443 953.0769 1 2.8209 109.9800 668.6900 2 2.5888 83.5278 361.3077 3 2.1492 55.0012 129.6923 4 1.9868 42.3300 91.3077 5 1.4314 8.0253 24.5385 6 0.8451 1.7485 8.9231 TABLE 2. Log-transformed MPN values for sites 1 to 13 Time Site 1 2 3 4 5 6 7 8 9 10 11 12 13 0 3.04 3.04 3.04 3.04 3.04 3.04 3.04 2.80 3.04 2.80 2.89 2.89 2.89 1 2.38 1.97 3.04 3.04 3.04 3.04 3.04 2.78 2.89 2.77 2.54 2.20 2.48 2 2.38 1.36 2.89 2.89 2.78 2.83 2.88 2.10 2.18 2.19 2.05 1.77 2.40 3 1.97 1.88 1.98 2.05 2.15 1.77 2.89 1.90 1.83 1.94 1.73 1.36 1.20 4 1.63 1.59 1.38 1.71 2.37 1.76 2.75 1.42 1.52 1.79 1.18 1.36 1.15 5 0.60 1.32 1.23 1.30 1.34 1.56 2.07 0.95 1.18 1.34 1.23 0.95 1.00 6 0.00 0.48 0.95 1.04 1.36 1.00 1.28 0.95 0.45 0.85 1.00 0.30 0.60 9 TABLE 3. Confirmatory results showing the presence and absence of E. coli after every hour for all sites, ‘+’ indicate the presence of E. coli and ‘-‘indicates the absence of E. coli Site Time 0 1 2 3 4 5 6 1 + + + + + 2 + + + + + + 3 + + + + + + 4 + + + + + + 5 + + + + + + 6 + + + + + 7 + + + + + + + 8 + + + + + + 9 + + + + + + 10 + + + + + 11 + + + + + 12 + + + + + + 13 + + + + + - 10 All the wells sampled were faecally contaminated as indicated by the presence of E. coli. E.coli was undetectable in 8 out of 13 (62%) and 12 out of 13 (92%) sites sets after 5 & 6 hours of exposure respectively (Table 4). TABLE 4. MPN values/100ml of coliforms for the two replicates (range and mean value in parentheses) and E. coli data (indicated by +). Site 1 2 3 4 5 6 7 8 9 10 11 12 13 0 hrs 1100-1100 (1100) 1100-1100 (1100) 1100-1100 (1100) 1100-1100 (1100) 1100-1100 (1100) 1100-1100 (1100) 1100-1100 (1100) 1100-150 (625) 1100-1100 (1100) 1100-150 (625) 1100-460 (780) 1100-460 (780) 1100-460 (780) 1hrs 240-240 (240) 93-93 (93) 1100-1100 (1100) 1100-1100 (1100) 1100-1100 (1100) 1100-1100 (1100) 1100-1100 (1100) 1100-93 (597) 1100-460 (780) 1100-75 (588) 460-240 (350) 240-240 (240) 460-150 (305) 2 hrs 240-240 (240) 23-23 (23) 460-1100 (780) 460-1100(780) 93-1100(597) 240-1100(670) 400-1100 (750) 210-43 (127) 150-150 (150) 290-21 (156) 150-75 (113) 43-75 (59) 43-460 (252) MPN/100ml 3 hrs 93-93 (93) 75-75 (75) 39-150 (95) 15-210 (113) 43-240 (142) 43-64 (59) 460-1100 (780) 150-9 (80) 93-43 (68) 160-15 (88) 15-93 (54) 23-23 (23) 9-23 (16) E. coli 4 hrs 43-43 (43) 39-39 (39) 9-39 (24) 9-93 (51) 11-460 (236) 20-93 (57) 23-1100 (562) 43-9 (26) 43-23 (33) 120-4 (62) 21-9 (15) 23-23 (23) 9-23 (16) 5 hrs 4-4 (4) 21-21 (21) 0-17 (9) 0-20 (10) 4-39 (22) 7-64 (36) 23-210 (117) 14-3 (9) 15-15 (15) 39-4 (22) 4-29 (17) 9-9 (9) 4-15 (10) 6 hrs 0-0 (0) 3-3 (3) 0-9 (5) 0-11 (6) 0-23 (12) 4-15 (10) 9-28 (19) 0-9 (5) 9-4 (7) 7-0 (4) 4-15 (10) 0-4 (2) 4-4 (4) + + + + + + + + + + + + + 11 Of the physico-chemical parameters recorded pH ranged from 6.5 to 8.2 and dissolved oxygen (DO) from 4 to 9.7. Conductivity was relatively uniform ranging from 205 to 342. The turbidity of most of the sites was below 5 NTU (Nephelometric Turbidity Units) except for site 7 which was 14. The water temperature recorded was almost uniform without significant difference and it ranges from 20.3 to 22.8. The physicochemical results are shown in Table 5. TABLE 5. Variation of physicochemical variables in the study sites. Site pH Conductivity (µS/cm) DO (mg/l) Water T°C Air T°C Turbidity (NTU) 1 6.77 299 6.7 21.8 19.1 1 2 6.57 247 5.4 21.7 22.9 1 3 6.97 245 5.6 21.2 19.4 1 4 7.01 342 6.1 21 19.4 1 5 6.9 341 6.4 20.7 17.6 1 6 8.07 211 6.1 20.3 19.5 2 7 8.22 225 6.4 21.5 25.8 14 8 8.03 205 5 20.4 21.5 4 9 7.72 247 9.7 22.8 22.7 2 10 7.83 242 5.8 22.1 21.6 2 11 7.71 243 5.3 22.8 22.6 3 12 7.68 246 4 22.7 21.7 2 13 7.69 237 4.3 22.5 21.5 2 A pH gradient seemed to exist in the study area (Table 5), with sites having pH values in the same range being clustered together geographically, and these clusters were classified into three groups A, B and C (Table 6, Figure 15). The clusters seemed to have characteristic coliform inactivation. TABLE 6. Mean MPN/100ml for coliforms and pH values for clustered sites, Group A (sites 1,2,3,4 & 5), Group B (sites 6, 7& 8) and Group C (sites 9,10,11,12 & 13).The number in brackets are standard deviations of the mean values of the grouped sites. Group 0hrs 1hr 2hrs 3hrs 4hrs 5hrs 6hrs Mean pH A 1100 (0) 727 (513.9) 548 (339.1) 135 (25.3) 50 (88.5) 15 (7.7) 6 (4.7) 6.8 B 942 (274.2) 932 (290.4) 526 (339.0) 305 (410.3) 2 15 (300.9) 68 (56.2) 11 (7.1) 8.1 C 813 (173.9) 436 (225.3) 146 (70.7) 50 (30.3) 23 (71.4) 15 (5.3) 2 (3.1) 7.7 y=9.80-2.91x P=0.002 2 r =87.9% y=9.80-2.91x 3.5 2 r =87.9% 3.5 3.0 3.0 2.5 2.5 LogMPN/100ml LogMPN/100ml P=0.002 2.0 1.5 1.0 2.0 1.5 1.0 0.5 0.5 0.0 0 1 2 3 4 Time (hrs) Fig. 15 (a) 5 6 7 0.0 0 1 Fig. 15 (b) 2 3 4 Time (hrs) 5 6 7 12 y=7.14-2.36x P=0.000 2 r =97.5% 3.5 3.0 LogMPN/100ml 2.5 2.0 1.5 1.0 0.5 0.0 0 1 2 3 4 Time (hrs) 5 6 7 Fig. 15 (c) Group C comprised sites with a mean pH value of 7.7 and appeared to have the highest rate of coliform inactivation having a total of two MPN value after 6 hours, followed by Group A with a mean pH of 6.8 and an average MPN of six after 6 hours, and Group B with a mean pH of 8.1 and MPN value of 11 after 6 hours. From the graph (Figure 15), the highest rate of decay of coliforms is shown by sites in Group C followed by Group A and lastly Group B. Re- growth of E. coli was also tested during the study. The results showed revival of coliforms at some sites and no revival at others (Table 8). TABLE 8. Total coliform MPN values from the re-growth investigation Site 1 2 3 4 5 6 7 8 MPN after 6hrs of 0 3 9 6 12 10 19 5 exposure t o the sun MPN after 24hours 4 0 11 15 4 0 28 15 storage in dark 9 7 10 4 11 10 12 2 13 4 4 15 9 0 4 13 Discussion This study confirmed that coliforms, notably E. coli are susceptible to solar disinfection upon adequate exposure to natural sunlight in field conditions. Escherichia coli were undetectable after the last hour of exposure in 92.3% of the samples tested (Table 3). After 5 hours of exposure, only 38.5% of the samples indicated total inactivation of E. coli. This confirms that six hours is the minimum time required to completely inactivate the E. coli under the conditions in this study. Site 7 was an exception with E. coli still present after 6 hours of exposure to sunlight. This correlated with the highest turbidity, an attribute that is known to reduce the disinfection efficacy of SODIS process. It has been reported that SODIS may be effective at turbidities as high as 200NTU (Kehoe S. C. et al, 2000). The findings in this study however indicated that field conditions may limit this, and turbidity may well remain an important factor in the application of SODIS. However, it appears that as expected the rate of inactivation under these condition is dependent on many factors and the complex interactions between them. A key issue in the setting in this study appears to be the faecal contamination. All the 13 sampled sites appeared to be faecally contaminated. The presence of E. coli in all the wells can be explained in terms of different factors. The wells, though deep and sometimes covered at the surface were unprotected below ground. This exposed the water sources to possible contamination by seepage from the pit latrines in the area. The latrines in the area were shallow pit latrines located within the same residential stand (hence in close proximity) to the wells. Furthermore, the containers used for drawing water were always exposed to the external environment, and thus were also susceptible to contamination. On a larger (regional) scale, Hatcliffe Extension lies on a slope such that the effect of individual household sanitation may be overshadowed by regional through-flow and percolation of groundwater by contaminants from wider environs. The fecal contamination however appears to be influencing the broader physico-chemical characteristics of the water. The most noted characteristic from this study was the pH. pH usually depends on the natural geology and the chemical composition of the soil (Water Quality Association, 2007), and physicochemical results from this study showed a possible localized clustering of sampling sites along a pH gradient. Although it is known that bacterial species metabolize efficiently at particular optimal pH ranges (Prescott et al., 1996), the effect of pH on the process of SODIS is not yet investigated. It could however, be an important determinant in the survival of coliforms during solar disinfection, hence the need for further investigation, possibly using multivariate techniques such as cluster analysis. The turbidity measured from the wells was in the expected range for efficient SODIS process with the highest extreme value recorded at site 7 (14 NTU), where the well was not covered at the surface. Turbidity possibly had an effect at this particular site since the rate of bacterial inactivation was relatively low (Fig 8) and a notable number of coliforms were still present in the water sample after 6 hours of exposure. The trend of coliform decay for each site followed an exponential decline that is typical of bacterial destruction by chemical disinfectants like chlorine (Acra et al., 1984). Re-growth of coliforms in priorly-exposed water samples produced inconsistent results among different sites, hence no substantive conclusions could be made as to whether SODIS can completely destroy microbial pathogens or it will just inactivate/disinfect them. Re-growth was seen in 42.2% of the samples whilst 53.8% of the samples showed no increase, indicating that the bacteria had been irreversibly inactivated in these samples. 4% of the samples showed a decrease in the number of coliforms after a 24-period in darkness and this can be accounted for by natural death of coliforms. However, some recent studies showed that E. coli could not grow outside the human or animal gut (Health Canada, 2008), thus casting doubts on the theory behind the concept of testing re-growth in artificial media. The observed re-growth could therefore have been caused by contamination after the disinfection process. There is need for further studies to investigate the re-growth dynamics at various times of exposure as there is possibility of inadequate exposure in field application. Thus the potential efficacy failure rates of the use of the method need to be quantified. A sample size of 13 out of over 2000 households was not enough to represent the whole area hence a large sample size would improve the resolution of the results. More replicates were important in order to improve the precision of the experiment and to increase the significance of statistical tests used. Since the process of SODIS depends on light intensity and temperature, measurements of these variables would have been important to the results obtained. The sampling period was not long enough to include effects of seasonal 14 variation on SODIS, but the results obtained pave way for further investigations on impact of pH on the SODIS process. It is recommended that SODIS can be used as an inexpensive, effective and applicable method of increasing drinking water safety in resource-limited countries such as sub-Saharan Africa. However, recommendations for its use must be accompanied by clear emphasis to ensure that the water is adequately exposed. Conclusions In conclusion, the process of SODIS can effectively reduce coliform bacterial counts in drinking water. However, research focus should be directed towards some predisposing factors such as the period of exposure, intensity of sunlight and climatic conditions (e.g. seasonal effects) in order to optimize the process. Environmental factors affecting the quality of water are also vital as explanatory variables to the overall efficacy of SODIS. The field application of the process must be appropriately guided as inappropriate practice could easily undermine the expected benefits from the process. ACKNOWLEDGEMENTS This study was derived from the broader SODIS water project Solar Disinfection of Drinking Water for Use in Developing Countries or in Emergency Situations (Sodiswater) being carried out by the Institute of Water and Sanitation Development (IWSD) in Harare with support from the European Union. We are grateful to IWSD and UZ Biological Sciences technical staff and students for their assistance during this investigation. 15 References ACRA A, RAFFOUL Z and KARAHAGOPIAN L (1984) Solar Disinfection of Drinking Water and Oral Rehydration Solution. Guidelines for Household Application in Developing Countries. UNICEF. Beirut. AMNESTY INTERNATIONAL, (2006) “Document: Zimbabwe: No Justice for the Victims of Forced Evictions”, (www.amnesty.org/fr/library asset; May 2006). BAILEY, N.T.J. (1995). Statistical Methods in Biology. 3rd edition. Cambridge University Press, UK. BAILEY, W.R and Scott, E.G. (1982). Diagnostic Microbiology. 7th edition. Mosby. St Louis. BRISCOE J AND VAN DER SLICE (1993). Water Supply and Health in Developing Countries: Selective Primary Health Care Revisited. American Journal of Public Health 74 22-28. CAIRNCROSS, S. AND FEACHEM, R. (1993). Environmental Health Engineering in the Tropics. 2nd edition. John Wiley and Sons Ltd. New York. CHAPMAN, D. (1998). Water Quality Assessment. 2nd edition. E and FN Spon, an Imprint of Routledge. New York. CLASEN, T. and Cairncross, S. (2004). Household Water Treatment: refining the dominant paradigm. J. Trop Med. Hyg. 9: 56-72 COLLIGAN, B., Couilliot M., Crepin X. and Duchemin J. (1999). Water Supply and Sanitation in Peri-Urban areas and Small Centres. Dumas. France. CONROY R.M, MEEGA M.E, JOYCE T, MCGUIGAN K and BARNES J. (1999). Solar Disinfection of Water Reduces Diarrhoeal Diseases: An update. Arch-Dis Child. 81: 337-338 EAWAG (SWISS FEDERAL INSTITUTE FOR ENVIRONMENTAL SCIENCE AND TECHNOLOGY). (2007). Solar Water Disinfection: A Water Treatment Process Used at Household Level. Dubendorf. Switzerland. ENVIRONMENTAL CONTROL AND PUBLIC HEALTH: COURSE TEAM. (1995). Water Supply and Sewage Treatment. The Open University, UK. FEACHEM R, BRADLY D, GARELICK M, and MARA D. (1983). Sanitation and Disease, Health Aspects of Excreta and Waste Water Management. John Wiley and Sons. UK. GORCHEV, H.G. (1988). Drinking Water Quality and Health. Grosvenor Press International. Hong-Kong. HEALTH CANADA. (2008). Guidelines for Canadian Drinking Water. Environmental and Workplace Health. Canada. JOYCE T.M, MCGUIGAN K.G, MEEGA M.E and CONROY R.M. (1995). Inactivation of Faecal Bacteria in Drinking Water by Solar Heating. Appl. Env. Microbiol. 62: 399-402 KEHOE S.C, JOYCE T.M, IBRAHIM P, GILLESPIE J.B, SHAHAR R.A and MCGUIGAN K.G. (2000). Effect of Agitation, Turbidity, Aluminium Foil Reflectors and Container Volume on the Inactivation Efficiency of Batch- Process Solar Disinfectors. Wat. Res. 35: 1061-1065. MAYA R.S. (1999). Handbook on Consumer Water Quality and Conservation in Zimbabwe. Southern Centre for Energy and Environment. Zimbabwe. MORELLO J.A, MIZER H.E, WILSON M.E and GRANATO P.A. (1998). Microbiology in Patient Care. McGraw Hill Companies. Inc. USA. PRESCOTT L.M, HARLEY J.P. and KLEIN D.A. (1996). Microbiology. 3rd edition. WCB Publishers, Dubuque, USA. SOBSEY M.D. (2002). Managing Water In The Home: Accelerated Health Gains From Improved Water Supply. WHO. Geneva. (http//www.who.int/water sanitation health/dwq/wsh 0207/en). SPECTRUM ITS. (2008). Cholera Threats in Hatfield and Hatcliffe. Combined Harare Residents Association. Zimbabwe. SZEWZYK, U, SZEWZYK, R, MANZ, W and SCHLEIFER, K.H. (2000). Microbiological Safety of Drinking Water. Annu. Rev. Microbiol. 54: 81-127. WATER QUALITY ASSOCIATION. (2007). Excel Water Technologies inc, Canada. WEGELIN M, CANONICA S, MECHSNER K, FLEISCHMANN T, PESARO F and METZLER A. (1994). Solar Water Disinfection: Scope of the Process and Analysis of Radiation Experiments. J. Water SRT Aqua. 43: 154-169 WHO (2003). Household Water Treatment and Safe Storage, Following Emergencies and Disasters. Geneva. 16 WRIGHT, J, GUNDRY, S and CONROY, R. (2004). Household Drinking Water in Evaluation of In-home Flocculation and Chlorination in Rural Guatemala. J. Water Health 46: 5-22. ZIMBABWE LAWYERS FOR HUMAN RIGHTS; Department of Sociology (UZ) (2005) “Report on the Impact of Operation Murambatsvina: The Case of Hatcliffe Extension, (http://www.zlhr.org.zw; May, 2008). ZWANE, A.P and KREMER, M. (2007). What Works In Fighting Diarrhoeal Diseases In Developing Countries? A Critical Review. Oxford University Press. California. Joyce TM, McGuigan KG, Elmore-Meegan M, Conroy RM. Inactivation of faecal bacteria in drinking water by solar heating. Apple Environ Microbiol 1996;62(2):399-402. Kehoe SC, Barer MR, Devlin L, McGuigan KG. Batch process solar disinfection is an efficient means of disinfecting drinking water contaminated with Shigella dysenteriae Type I. Lett Appl Microbiol. 2004;38(5):410-414. Smith RJ, Kehoe SC, McGuigan KG, Barer MR. Effects of simulated solar disinfection on infectivity of Salmonella typhimurium. Lett Appl Microbiol 2000; 31(4):284-288. Lonnen J, Kilvington S, Kehoe SC, Al Touati F, McGuigan KG. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Research. 2005;39(5):877-883. WHO, 2008-GLAAs report 17 List of Figures Figure 1. Average number of coliforms for all sites after an hourly interval of coliform test. The scatter plot indicates that the number of coliforms decreases after every hour and the error also follow the same trend. Figure 2. Rate of coliform decay in water collected at site 1 Figure 3. Rate of coliform decay in water collected at site 2 Figure 4. Rate of coliform decay in water collected at site 3 Figure 5. Rate of coliform decay in water collected at site 4 Figure 6. Rate of coliform decay in water collected at site 5 Figure 7. Rate of coliform decay in water collected at site 6 Figure 8. Rate of coliform decay in water collected at site 7 Figure 9. Rate of coliform decay in water collected at site 8 Figure 10. Rate of coliform decay in water collected at site 9 Figure 11. Rate of coliform decay in water collected at site 10 Figure 12. Rate of coliform decay in water collected at site 11 Figure 13. Rate of coliform decay in water collected at site 12 Figure 14. Rate of coliform decay in water collected at site 13 Figure 15. Rate of coliform decay observed in (a) Group A (b) Group B and (c) Group C Figure 16. Rate of coliform destruction over a period of 6 hours. 18