View the Resource
advertisement

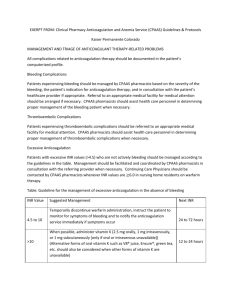
HANYS’ 2010 Pinnacle Award for Quality and Patient Safety Submission Template and Guidelines Section I: A. Contact information required for publication and feedback. Required Information Please type in white space only. Complete below: Full Name Abdul Mondul MD Credentials Title Assistant Professor of Clinical Medicine Weill Medical College at Cornell University Organization Name Patient Safety Officer Associate Chair Department of Medicine Organization Address Lincoln Medical and Mental Health Center 234 East 149th Street Bronx, New York 10451 Telephone E-mail 718-579-5280 Abdul.Mondul@nychhc.org B. Check applicable submission category: [ ] System or multi-level entity [X] Large Hospital (> 100 mean daily patient census) [ ] Small Hospital or Outpatient Organization [ ] Division, Specialty, or Unit-based entity C. Check if you are a Nassau-Suffolk Hospital Council (NSHC) member and would like this entry to also be submitted for the NSHC Annual Quality Award. [ ] Yes Section II: See brochure for directions. This project narrative section should be (1) written and reviewed for potential publication, (2) must not include any facility-identifying information for the judges’ review, and (3) cannot exceed this one page format using just the white space section, single spaced, and11 pt. Times New Roman. Information Required Please Complete in This Column Name of Initiative This section is between 25 and 30 words. Project Description (Narrative Summary) This section is between 150 and 225 words, depending on use of space and bullets. Improving Anticoagulation Safety Through a Hospital Wide, Interdisciplinary Approach Anticoagulants are high-risk, high-alert and high-value medications. Management of these drugs is a complex and challenging task for health care systems. These live saving medications are used to treat and prevent arterial and venous thrombo-embolism; however they are also frequently associated with medication errors. A systematic interdisciplinary review of our anticoagulation practices was conducted, opportunities for improvement were identified and specific indicators to measure process and outcomes were developed. Methods used included failure mode effects analysis, GAP analysis, literature review, expert team meetings and data collected prior to program implementation. Based on the findings a hospital wide anticoagulation program was implemented with strategies to improve the safe use of Warfarin, Low molecular weight Heparin and un-fractionated heparin. An anticoagulation manual was developed to facilitate hospital wide education and competency evaluation. An electronic order set embedded within the CPOE (computerized physician order entry) was implemented to provide additional clinical decision support. A patient registry was created for patients on anticoagulation that follow in our ambulatory care clinic and system changes were implemented on the tracking, recall and visit structure for them. Point of care testing for INR was also adopted. There is ongoing monitoring of the indicators and these are shared in several hospital committees. A New York State DOH Empire Clinical research investigator program (ECRIP) scholarship that will focus on anticoagulation was applied for and granted. Outcomes Achieved Please use bullets A one-page Word document can also be submitted containing one or more graphics. This section is approximately 60 words when using bullets. Lessons Learned Top three lessons utilizing bullets. The number of patients within therapeutic INR in our ambulatory care clinic increased from 50% to 83 % (Literature reference 55 -60%) Compliance with weight based dosing of Unfractionated heparin initial bolus improved from 73% to 100% and for maintenance from 80% to 95% Compliance with weight based dosing of Low molecular weight heparin in the inpatient services increased from 80% to 95 % Compliance with protocol defined transition from Unfractionated heparin or Low molecular weight heparin to warfarin increased to 95% (Literature search reference 50%) Education by dietitian of patients on Warfarin in the inpatient units increased from 69% to 97% Unmonitored dose reduction of Warfarin (When the dose of Warfarin is reduced and an INR is not repeated prior to resuming therapy) decreased to zero Implementing a comprehensive anticoagulation program requires interdisciplinary commitment, collaboration and ongoing education Developing process and outcomes measures is important to address successes and opportunities for improvement Our hospital is performing better than the available published data on multiple anticoagulation indicators and significant improvement was achieved through this program learned This section is approximately 45 words when using bullets. Section III: Additional supporting information cannot exceed two pages. Please do not include identifying information for judges’ review. Information Required Problem Statement Please Complete in This Column Anticoagulants are life-saving drugs used to treat and prevent arterial and venous thrombo-embolism; however they are also a high-risk high-alert category of drugs that are frequently associated with medication errors The Institute for Safe Medication Practices (ISMP) and the Institute for Healthcare Improvement (IHI) emphasize the importance of a comprehensive anticoagulation program and in 2008 The Joint Commission issued NPSG 3E that aims to reduce the likelihood of harm associated with the use of anticoagulants. In patients taking warfarin, maintaining an INR within the therapeutic range is difficult. Patients within a clinical trial setting had therapeutic INR’s only 60% of the time and a recent review revealed that clinic patients who receive long-term Warfarin achieve therapeutic INR’s only 55% of the time. Having INR’s out of therapeutic range 10% of the time is associated with an increased risk of mortality, ischemic stroke and other thromboembolic events among those receiving long- term Warfarin for non-valvular atrial fibrillation. Warfarin is commonly associated with adverse events, mainly bleeding due to its narrow therapeutic window (i.e., it is easy to over or under dose), the complexity of dosing and monitoring, patient compliance, numerous drug interactions, and dietary interactions. Errors related to Unfractionated heparin accounts for majority (66.2%) of errors or 1.67 medication errors for every 1,000 patients receiving anticoagulation therapy (Franikos, et. al, 2004). 6.2% patients that were exposed to anticoagulant medication error required some type of medical intervention, while 1.5% required prolonged hospitalization. Strict compliance with Weight based dosing of unfractionated heparin and low molecular weight heparin, protocol guided transition to warfarin and protocol guided chronic warfarin therapy reduces the risk for adverse outcomes. Aim-Goals Methodologies and Change Principles Standardize and implement best practices in the prescribing, administering and monitoring processes in the use of un-fractionated heparin, low-molecular weight heparins and Warfarin Ensure effective education, interaction, and collaboration among care providers and patients to achieve therapeutic levels of anticoagulants and maintain safety during use Develop Indicators to measure effectiveness of the anticoagulation program Baseline evaluation of anticoagulation practices by the Pharmacy & therapeutics and Drug utilization – Evaluation committees (2006 - 2007) Formed a multidisciplinary anticoagulation practices committee (2007) Incorporated dosing guidelines as a Clinical decision tool in the EMR (2007) Failure mode effects analysis on anticoagulants conducted ( 2007) GAP analysis and implementation of 20 best practices recommended by ISMPIHI (2007) Developed a system to generate a daily list of all Inpatients on anticoagulation for referral to dietitian (2007) Compiled a comprehensive Anticoagulation manual reflecting best practices for clinicians (2008). This manual was rolled out to other hospitals in our corporation (2009) Developed Patient education tool and translated into Spanish (2008); the educational tool was then translated into the top 12 languages of our patients (2009) Additional Outcomes Not Listed in Section II Sustainability Strategies Educated all clinical staff on safe anticoagulation practices with specific elements and competencies ( Pharmacy, Nursing, Nutrition, Physicians) (2008) Implemented an electronic order embedded within the CPOE (computerized physician order entry) (2008) Created a patient registry and recall system for patients on anticoagulation in the ambulatory care and INR point of care testing (2008) 100% review of the records of patients that receive therapeutic heparin (both Unfractionated and low molecular weight) Developed Multiple Performance improvement projects to measure and share success One of the achievements of this project was increased staff knowledge and understanding of their significant role in the dosing, monitoring and patient education as it relates to anticoagulation therapy. Residents, nurses, pharmacists and dieticians know the risks and benefits of anticoagulation therapy. The use of an outpatient registry to track, recall and a streamlined visit structure for anti-coagulated patients contributed to an excellent rate of therapeutic INR in the out patient setting Continuous monitoring of indicators and reporting to the Drug utilization / evaluation committee and the hospital wide performance improvement committee Pharmacy concurrent monitoring of anticoagulation practices. All deviations from protocols are reported to the patient safety officer who in turn provides feedback to the chiefs of services and providers Hospital wide anticoagulation education with yearly competency assessment specific to clinical discipline Integration of performance improvement with research through the New York State DOH Empire Clinical research investigator program (ECRIP) scholarship Business Case Information Conclusion, Recommendation s, or Next Steps Celebrating success: for example the pharmacists involved in the project were acknowledged as patient safety champions during National Patient Safety Week, March 2010 It is known that 1.5 million preventable adverse drug events (ADE’s) occur in the United States each year, over 770,000 people are injured or die each year in hospitals from ADE’s at an annual cost up to $5.6 million per hospital and total cost to US $5.6 billion annually. Warfarin and insulins caused: one in every seven ADE’s treated in emergency departments and more than a quarter of all estimated hospitalizations. In the elderly, insulin, warfarin, and digoxin were implicated in one in three estimated ADR’s treated in the emergency departments and 41.5% of estimated hospitalizations. Patients that were exposed to anticoagulant medication error from unfractionated heparin required some type of medical intervention and prolonged hospitalization. The implementation of a comprehensive anticoagulation program reduces costs related to treatment, readmission or prolonged hospital stay related to ADR’s. It is important to utilize a multi-disciplinary approach to improve the safe use of high alert medications by implementing best practices, adopting performance indicators and developing educational tools for providers and patients Excellent compliance with protocol guided anticoagulation practices is achievable Going forward, we will continue to monitor performance, ongoing clinical staff education, share our processes with other hospitals in our corporation and publish our data with the support of the ECRIP research fellow. A. Patients with Therapeutic INR in Ambulatory Care 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% B. Low Molecular Weight Heparin Dosing 100% 80% 60% 40% 20% Published Reference 1st Qtr 2007 11 /0 8 2 11 /08 007 ( n 12 =1 /08 3) (n= 1/0 15 ) 9( n= 2/0 12 ) 9( n= 3/0 1 4) 9 4/0 (n=9 ) 9( n 5/0 =1 0 ) 9 (n 6/0 =4 ) 9( n 7/0 =9 ) 9(n 8/0 =4) 9(n 9/0 =8) 9(n 10 /09 =8) (n= 1 11 /09 5) (n= 12 /09 6 ) (n= 8) 100 90 80 70 60 50 40 30 20 10 0 F. Dosing of Un-fractionated Heparin Infusion 100 90 80 70 60 50 40 30 20 10 0 11 /0 200 8 7 12 (n /0 = 1 8 5) 1/ (n= 09 16 ) 2/ (n= 09 1 5 (n ) 3/ =1 09 5 ) 4/ (n 09 =9 (n ) 5/ =1 09 0) 6/ (n= 09 7 ) ( 7/ n=9 09 ) ( 8/ n=6 09 ) 9/ (n= 09 9 ) 10 (n= /0 11 9( ) 11 n=1 /0 7) 12 9 (n /0 =8 9( n= ) 11 ) E. Dosing of Un-fractionated Heparin Bolus 4th Qtr 2009 =4 ) 11 /0 9( n= 4) 4th Qtr 2009 =2 ) 3rd Qtr 2009 =4 ) 2nd Qtr 2009 (n 1st Qtr 2009 3rd Qtr 2009 9/ 09 (n Published Reference 4th Qtr 2008 2nd Qtr 2009 100 90 80 70 60 50 40 30 20 10 0 3) 40% 3rd Qtr 2008 1st Qtr 2009 7/ 09 (n 60% 2nd Qtr 2008 4th Qtr 2008 (n = 80% 0% 3rd Qtr 2008 D. Appropriate Transition from Un-fractionated Heparin to LMWH / Warfarin 100% 20% 2nd Qtr 2008 3) C. Low Molecular Weight Heparin Transition to Warfarin 1st Qtr 2007 0% Nov09 5/ 09 Sep09 (n = Jul09 4) May. 09 3/ 09 Mar. 09 (n = Jan. 09 1/ 09 Nov. 08 G. Inpatients on Warfarin - Education by Dietitian 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0% Sept. 08 4th Qtr 2008 1st Qtr 2nd Qtr 3rd Qtr 2009 2009 2009 H. INR Availability at Follow up Appointment 4th Qtr 2009 A one-page Word document containing one or more graphics can also be submitted.