write up for webpage..
advertisement
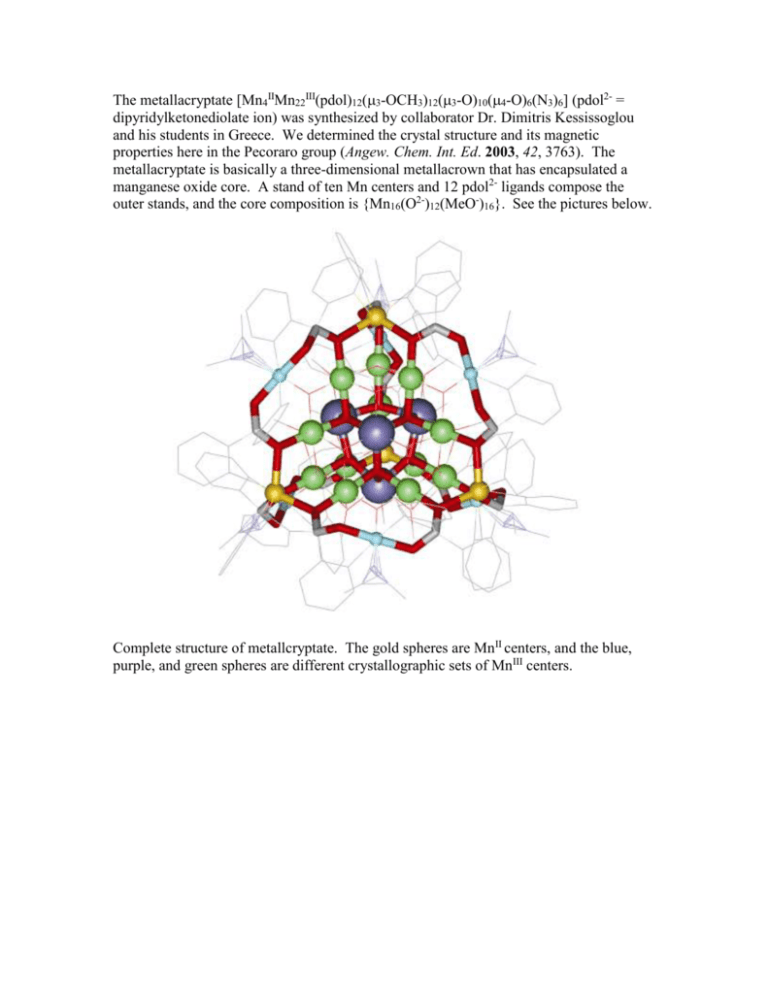
The metallacryptate [Mn4IIMn22III(pdol)12(3-OCH3)12(3-O)10(4-O)6(N3)6] (pdol2- =
dipyridylketonediolate ion) was synthesized by collaborator Dr. Dimitris Kessissoglou
and his students in Greece. We determined the crystal structure and its magnetic
properties here in the Pecoraro group (Angew. Chem. Int. Ed. 2003, 42, 3763). The
metallacryptate is basically a three-dimensional metallacrown that has encapsulated a
manganese oxide core. A stand of ten Mn centers and 12 pdol2- ligands compose the
outer stands, and the core composition is {Mn16(O2-)12(MeO-)16}. See the pictures below.
Complete structure of metallcryptate. The gold spheres are MnII centers, and the blue,
purple, and green spheres are different crystallographic sets of MnIII centers.
Stereoview of the stands of metallacryptate with the core removed. The color scheme is
the same as above.
Stereoview of the core of the metallacryptate. Same color scheme as above.
The ac magnetic susceptibility data indicate that this molecule behaves as a singlemolecule magnet (SMM). As the in-phase susceptibility decreases near 2.5 K, the out-ofphase susceptibility increases (a characteristic signal of SMM’s). Unfortunately, a
blocking temperature could not be determine just yet since the limit of the AC SQUID
used in the study is 2.0 K. Future work at lower temperatures is in progress. The dc
magnetic susceptibility data indicate that an overall antiferromagnetic interaction
dominates in the cluster, however a ground state could not be determined since the
magnetization does not saturate at 2K from 100 G to 55, 000 G. Work at lower
temperatures and higher magnetic fields is in progress. Single-molecule magnets are a
relatively new class of molecules (first identified in 1993 by Christou and coworkers) that
show both classical and quantum effects and may have applications in memory storage.
The ac susceptibility data indication SMM behavior.