5.1.09 mends trial

THE MENDS TRIAL
EBM
Serena Ezzeddine
May 1,2009
Effect of Sedation With Dexmedetomidine vs Lorazepam on Acute Brain
Dysfunction in Mechanically Ventilated Patients . The MENDS Randomized
Controlled Trial.
Clinical Question: Besides Lorazepam, is there another agent that can or is used for sedation?
Background:
Sedative and analgesic medications are routinely administered to mechanically ventilated patients to reduce pain and anxiety and to allow patients to tolerate invasive procedures in the ICU. Unfortunately these medications can increase mechanical ventilation time, ICU stay, and development of acute brain dysfunction.
Delirium is an independent risk factor for prolonged length of stay.
Lorazepam is currently recommended by the Society of Critical Care Medicine
(SCCM) in its clinical practice guidelines for the sustained sedation of mechanically ventilated ICU patients.
Although the mechanisms by which benzos predispose patients to delirium is not proven, they act on GABAa central nervous system receptors that alter potential deliriogenic neurotransmitters, such as DA, Serotonin, acetylcholine, norepi, and glutamate.
Dexmedetomidine is a highly selective alpha 2 adrenergic receptor agonists that acts at the locus ceruleus and spinal cord to produce both sedation and analgesia, and is a viable yet understudied alternative to GABA-mimetic sedatives.
Hypothesis: Dexmedetomidine, when compared with benzodiazepine drugs, reduces the duration of delirium and coma while effectively sedating mechanically ventilated ICU patients.
This was an Investigator-initiated study in which the authors obtained an investigational New Drug approval from the FDA. This approval permitted the study of dexmedetomidine for longer than the current 24-hour FDA-labeled indication for use in the ICU and at doses as great as 1.5ug/kg/hr, which is higher than the doses currently approved by FDA (less than or equal to 0.7 ug/kg/hr).
The Institutional review boards at Vanderbilt University and Washington Hospital
Center approved this study.
Validity
:
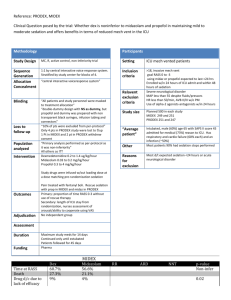
1. Were the patients randomized? Yes
Figure 1 i.
Inclusion Criteria-Adult Mechanical and Surgical ICU patients requiring mechanical ventilation for longer than 24 hours
ii.
Exclusion Criteria- excluded due to neurologic disease that would confound the diagnosis of delirium, active seizures, Childs Pugh class B or C liver disease, morbibund state with planned withdrawal, family or physician refusal, alcohol abuse, active myocardial ischemia, 2 nd
or 3 rd
degree heart block, severe dementia, benzo dependency, pregnancy/lactation, and severe hearing disabilities or inability to understand English.
Randomized using computer-generated permuted block randomization
(known only to the investigational pharmacists) and stratified by site to receive Lorazepam or Dexmedetomidine.
Detailed Info of sedative and analgesic meds given prior to randomization was obtained, and per FDA request, baseline EKG and endocrine levels were obtained prior to study drug administration
2.
Was follow-up of patients sufficiently long and complete? As good as can be expected considering ICU patients
Delirium measured until Hospital Discharge or for 12 days using
Confusion Assessment Method for ICU (CAM-ICU)
Patients were observed in the Hospital from enrollment until discharge or death or until study day 21, and survivors were observed for vital status until 1 year after enrollment, using EMR.
Also 28-day mortality was one of the secondary outcomes
3.
Were all patients analyzed in the groups to which they were randomized?
Yes. Figure 1
4.
Were the patients and clinicians and study personnel kept blind to treatment?
All patients and study personnel except investigational pharmacist were blinded to study drug assignment
5.
Were the groups similar at the start of the trial? Yes. Table 1
6.
Were the groups treated equally, apart from experimental treatment? Yes
Used infusion instead of bolus dosing to preserve blinding and minimize potential adverse effects.
Study drug was infused and titrated by the bedside nurse to a max infusion rate (10mg/hr Lorazepam or 1.5ug/kg/hr in Dex) to achieve the sedation goal set by the pt’s medical team using Richmond Agitiation-Sedation
Scale (RASS)
Study drug administered until extubation or for max time allowed by FDA
(120 hrs) and infusions could be stopped at any time when pt was at sedation target
Pts who were mechanically ventilated >120hrs were then sedated according to standard practice of the ICU
Apparent pain was treated with intermittent doses of Fentanyl, and was based on physiological parameters, ie BP, HR, RR, facial expressions, limb movement and ventilator synchrony. Continuous infusion of fentanyl was allowed
Allowed bolus propofol use of 25-50mcg if levels of agitation could cause harm to pt or staff
No open label use of both meds were allowed during study period
Daily awakening per ICU team
Pts underwent neuropsych testing within 72 hrs of ICU discharge as long as were CAM ICU negative, testing included Mini-Mental and Trails B
Cardiac safety profile –EKG, Trop on day 2, 4 and 2 days after drug discontinuation
Hormones measured at beginning were also measured 2 days after study drug stopped
Tbili and GGT at 2, 4 days and 1 week after discontinuation of study drug
OUTCOMES:
Primary Outcome
Delirium –free and Coma-free days, defined as days alive without delirium or coma.
-There was also evaluation of efficacy of the 2 sedation regimens in achieving clinically individualized target sedation goals
Secondary Outcomes
Length of stay with ventilation, in the ICU, and in the hospital, along with neurophysiological testing after ICU d/c, 28 day mortality, and 12 month survival from enrollment
Ventilator Free days were defined as number of days alive and not using mechanical ventilation over a 28-day period.
DEFINTIONS:
Delirium- RASS of -3 or greater (-2. -1, 0, etc) and positive CAM-ICU pg 2646
Coma- RASS of -4 or -5
Efficacy of the drug - Ability to achieve sedation score within 1 point of desired sedation goal- and was undertaken given both nurse and physician goal sedation scores.
Results: TABLE 2
-Dexmedetomidine patients had more days alive without delirium or coma, but if you look closely these are due to coma not delirium
-Nonsignificant differences were found in many of the secondary outcomes
-12 month time to death was 363 days in Dex group vs 188 days in Lorazepam, but likelihood of dying at 12 months was similar between groups
-Higher percentage of Dex patients were able to complete neuropsych testing but this was not a significant difference. The time from enrollment to testing was 2.5 days earlier in
Dex group (7 vs 9.5 days),which researchers felt reflected an earlier return to delirium negative cognitive state.
Dexmetedomidine
Lorazepam
Days w/Coma
2
4
Days w/o Coma
10
8
EER 2/12=0.17=17%
CER 4/12=.33=33%
RRR = (CER-EER)/CER= .33-.17/.33= .50=50%
When you dichotomize the outcome, then the difference seems to be a lot bigger than the numbers!
Efficacy of Sedation and Safety evaluation : TABLE 3, and FIGURE 4
-Pts with Dex spent more time at the level of sedation targeted by both nurses and physicians.
-But the median Fentanyl dose was 575 ug per day in Dex group vs 150 ug/day in
Lorazepam group!!!! (p=.006) ,and difference was more notable in those with deeper sedation goals ( Figure 5 )
- 7 pts in Dex group received propofol boluses for dangerous agitiation or procedures vs
4 in Lorazepam group
-No difference in anti-psycotic med administration
Table 4 - Safety Evaluation
- Sinus brady was more predominant in the Dex group (HR <60) , although only one patient from each group had an episode of brady <40, and none were associated with hemodynamic compromise
-4 self extubations in the Dex group vs 2 in Lorazepam group
-Dex group had nonsignificant increase in Afib (3 vs 0 pts in Lorazepam)
-No statistical difference in any lab tests
-Cost of Care was higher in Dex group vs Lorezapam, median cost approximately $22,
500 higher, but was not statistically significant and difference was thought to be due to costs prior to enrollment. Table 5
DISCUSSION:
-There is a statistically significant decrease in Coma, but no necessarily delirium. The primary outcome was coma free and delirium free days together, but the difference was solely due to Coma contribution
-Does not seem that Dex is an effective sedation tool- Why was so much Fentanyl needed? And, with the increased use of narcotics, are we not contributing to delirium?
Does Dex actually have a significant impact on analgesia? Do we need higher dosing to achieve sedation? Was there a 1 to 1 correlation between Lorazepam and Dex dosing to compare the two , ie were the max doses of the different drugs equivalent?
-What percentage of pts switched into the Lorazepam group?
-RASS scores were not standardized and were not reported for the separate groups
-Based on this study alone, would not change current sedation guidelines/practices, but has opened doors that will hopefully lead to more studies assessing better sedation tools that are less deliriogenic than current GABA acting drugs.