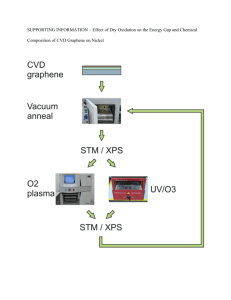
Experiment 3
advertisement

Experiment 3 Nickel Triphenylphosphine Complexes Nickel Triphenylphosphine Complexes - 1 Experiment 3: Preparation and Properties of Nickel Triphenylphosphine Complexes Aims to prepare complexes [Ni(PPh3)2X2] (X = Cl, Br, NCS) to elucidate their geometries using UV-visible spectroscopy and magnetic moments Introduction The coordination geometries of nickel(II) complexes are extremely sensitive to changes in the ligand set. For example, a number of complexes of the type [Ni(PR 3)2X2] exhibit a square planar to tetrahedral equilibrium in solution, a result of the very small energy difference between the two geometries. The geometry adopted depends on both electronic and steric effects. A more bulky phosphine ligand (e.g. tricyclohexylphosphine) will shift the equilibrium towards the tetrahedral form, whilst decreasing the bulk makes the square planar form more accessible. Similarly, stronger field ligands increase the tendency to a square planar geometry. PR3 R3P Ni R3P X X Ni R3P X X Experimental a) Preparation of Dichlorobis(triphenylphosphine)nickel(II) - Dissolve nickel chloride hexahydrate (1.2 g) in dry ethanol (15 cm 3) and warm gently. - Place triphenylphosphine (2.8 g) and isopropanol (30 cm 3) in a 100 cm 3 round-bottomed flask, fit a reflux condenser and dissolve the phosphine by refluxing gently. - When the phosphine has dissolved, remove from the heat and carefully add the warm nickel chloride solution. - Reflux the mixture for a further 10 minutes and then cool to room temperature. - Filter the product in a Buchner flask, wash with cold ethanol (15 cm 3 ) followed by diethyl ether (15 cm3) and draw a stream of air through the product to dry. - Record the yield (g , %). b) Preparation of dibromobis(triphenylphosphine) nickel(II) Use the same method as for the chloro-complex above but replace nickel chloride hexahydrate with nickel bromide trihydrate (1.4 g). c) Preparation of Dithiocyanatobis(triphenylphosphine) nickel(II) - Dissolve nickel nitrate hexahydrate (1.5 g) in ethanol (25 cm 3), with gentle warming if necessary, in a 100 cm3 round bottomed flask. - Add finely ground sodium thiocyanate (0.8 g), fit a reflux condenser and reflux for 20 minutes. - Meanwhile, prepare a triphenylphosphine solution in isopropanol as described in part (a) above. - When the reflux is complete cool the nickel thiocyanate solution on an ice bath whilst scratching the inside of the flask to induce crystallisation of sodium nitrate and unreacted sodium thiocyanate. - Filter off any solid and carefully add the filtrate to the triphenylphosphine solution. - Reflux for a further 10 minutes. - Cool the mixture on an ice bath. Nickel Triphenylphosphine Complexes - 2 - Filter off the product by suction, wash with cool diethyl ether (10 cm 3) and air dry. - Record the yield (g, %). d) Investigation - Measure the magnetic moments of each of the complexes. - Record the infra-red spectra of each of the complexes as nujol mulls. - Record the solution UV-visible spectra of each of the complexes. - Using the Cambridge Crystallographic Database search for nickel complexes of formula [Ni(PR3)2X2] where R is any organic group and X is any halide or pseudohalide (eg CN -, SCN-, N3etc). Questions 1. 2. 3. 4. 5. Calculate the effective magnetic moments of each of your compounds. What geometries do these results suggest for each of the compounds? Compare your UV-visible spectra. Do these support your conclusions on the basis of your magnetic measurements? Thiocyanate is an ambidentate ligand. From your infra-red spectrum deduce how it is bound to the nickel centre. Rationalise your results in terms of the donor properties of the ligands. Using your results from the crystallographic database, correlate the structures adopted (square planar or tetrahedral) with the type of halide and both the steric bulk and electron donor ability of the phosphines. Nickel Triphenylphosphine Complexes - 3