DIVISION OF INFECTION & IMMUNITY
advertisement

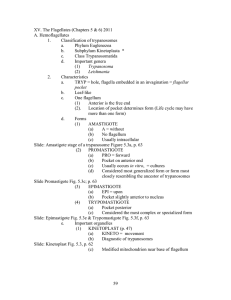
Standard Operating Procedure for Handling African Trypanosomes Wellcome Trust Centre for Molecular Parasitology For the purposes of the Control of Substances Hazardous to Health (COSSH) Regulations, pathogens are regarded as substances hazardous to health. This Standard Operating Procedure must be observed when working with any pathogenic trypanosomes. Trypanosoma brucei parasites are categorised in Hazard groups 2 and 3* according to ADCP guidelines. T. brucei, T. congolense, T. equiperdum, T. evansi and T. vivax are all Specified Animal Pathogens. All work on these parasites must be conducted in accordance with COSHH and the relevant Specified Animal Pathogen Order. All laboratory staff involved in handling these trypanosomes must have undergone appropriate training before starting work. Supervision must be at the level indicated on the COSSH Risk Assessment Form that relates to the procedure being carried out. All ancillary and maintenance staff must be informed of these rules. In particular, regarding the restrictions on access to the laboratories. Arrangements for access should be agreed by Head of Laboratory or local Safety Officer as appropriate. T. b. brucei, T. b. rhodesiense and T. b. gambiense bloodstream and metacyclic forms are considered as being infective to humans. Other stages of the life cycle (including the procyclic stage) are considered non-infective. Infection is unlikely to occur unless viable organisms are introduced into the body through the skin (or equivalent). Infection via inhalation or ingestion has not been reported and for this reason they can be handled on the open bench safely. Work need only be conducted in a microbiological safety cabinet for reasons of prevention of contamination of cultures. When handling viable organisms the following procedure must be followed; 1. Viable organisms must be restricted to appropriated areas of the Sir Graeme Davis Building (SGDB; 1/19, 1/28, 2/43, 5/35, 5/36, 5/37, 5/38, 5/39, 5/40, 5/42, 5/43, 5/44, 5/45, 5/47, 5/51, 5/53, 6/34, 6/35, 6/36, 6/37, 6/38, 6/39, 6/40, 6/41, 6/42, 6/43, 6/44, 6/45, 6/46, 6/47, 6/48, 6/54 and 6/55), Joseph Black Building (JBB; A2.15 and B2.16) or Cardiovascular Research Centre (CRC; C308 and C341). Use of trypanosomes and other pathogens must be separated in time or space, always. In particular, trypanosomes and other pathogens cannot be placed in the same incubator/water bath/centrifuge/microbiogical safety cabinet at the same time. 2. Admission to laboratories must be restricted to authorised personnel. 3. Appropriate protective clothing (i.e. disposable gloves, laboratory coats) must be worn. Cuts and scratches must be kept covered. Grey lab coats should be worn by workers handling live trypanosomes. The grey lab coats should not be worn outside of the trypanosome tissue culture rooms (5/42, 6/43 or 6/44) unless live trypanosomes are being handled in another laboratory. 4. Viable organisms must be kept in sealed containers whenever possible and be appropriately labelled. Viable organisms in unsealed containers must never be left unattended. Transfer of organisms between rooms and laboratories must always be in sealed containers. 5. Contaminated glassware, general apparatus and safety clothing should be decontaminated by immersion in 3% chloros, 3% trigene or 5% Bleach. Potentially contaminated lab coats must be autoclaved. Specialised equipment should be decontaminated by wiping with tissues soaked in chloros, trigene or Bleach. 6. Work on or over a tray whenever possible, work areas (e.g. bench tops) which may have become contaminated should be decontaminated with either 70% Ethanol, trigene or Bleach whenever necessary. 7. After clearing up, when leaving the lab and at other times as appropriate, workers should wash their hands well. 8. If a spillage of viable organisms occurs, the contaminated areas must immediately be swabbed with ethanol, chloros, trigene or Bleach. All accidents or potentially dangerous occurrences must be reported immediately to the Head of Laboratory or local safety Officer. 9. All potentially contaminated waste materials must be made safe before disposal. Normally, this will be in 3% chloros, 3% trigene or 5% bleach but used pipette tips can be put directly into dry waste containers where the trypanosomes will die by desiccation. The bags in these waste containers are sealed when full and autoclaved. All potentially contaminated plasticware is to be autoclaved. The bags in which they are placed to be sealed, boxed and transported by trolley to the autoclave in room 1/28. A record should be kept of the number of bags removed for autoclave - a book is provided for this purpose on each level of the building. All disposals should also be entered into the separate book that is located next to the autoclave. When work is carried out in rooms C308 and C341 of the cardiovascular building, or room 5/51 of the SGDB, SAPO waste should be transferred to rooms 6/43 or 6/44 for disposal. All equipment used in rooms C308, C341 and B5/51 should be decontaminated as described in point 5 10. Where trypanosomes have been used in experiments in conjunction with radioisotopes then material must be disposed of into dry waste containers, from which they will be dealt with as part of the radioisotopes waste. 11. In the event of any accident where trypanosomes may have had direct contact with the human body, wash the affected part immediately with copious volumes of water. If the skin is damaged, express blood from the wound and rewash. All accidents or potential dangerous occurrences must be immediately reported to the local Safety Officer or other appropriate authority and a ‘green form’ completed. Monitor for flu-like symptoms for eight weeks but do not take prophylactic drugs. In the event of flu-like symptoms, report to the local safety officer who will arrange for formal clinical diagnosis as appropriate. 12. Infected animals may be temporarily held in room B5/51(not overnight). Transfer of trypanosomeinfected animals between the JRF and the SGDB will be by secure van and animals must always be in cages with appropriately labelled cage cards. During transit, the cage lids must be taped down and the cages held in closed, Virkon sprayed bags. 13. Cage cards for cages containing animals infected with pathogens must be labelled with a red P. 14. Cage cards for cages containing animals injected with Cyclophosphamide must be labelled with a red C. 15. Gloves and lab coat should always be worn. 16. Live infected animals must not be left outside cages. The mouse barrier must be in place across the doorway. 17. Trypanosome-infected animals must be separated in time and space from use of other animals and pathogens. 18. If live animals are transferred from the JRF to the SGDB for procedural purposes and are euthanased within the SGDB, then under the Animal By-product Regulations, they cannot be transferred back to the JRF for disposal. SAPO cadavers must be double bagged, labelled as SAPO waste, stored within a designated freezer in the SGDB, and autoclaved. A record should be kept of the number of bags and amount of carcasses removed for autoclave - a book is provided for this purpose on each level of the building. All disposals should also be entered into the separate book that is located next to the autoclave. Autoclaved cadavers will be uplifted by Biological Services following the procedures detailed in Biological Services Standard Operating Procedure 37. 19. All bedding from infected mice must be double bagged with other decontaminated plasticware or tissues. Bags must be labelled as SAPO waste and transported to rooms 6/43 or 6/44 for disposal where it will be numbered and recorded for autoclaving. 20. When working with tsetse flies, observe and comply with the Standard Operating Procedure for handling tsetse flies. 21. Transfer of organisms between laboratories within SGDB must be in labelled, doubly sealed containers, such as within a sealed tube held in a sealed polythene box. 22. Transfer of trypanosomes between this and other laboratories must comply with IATA regulations. Packages containing trypanosomes will be handed only to approved staff and opened only in the designated laboratories. Packages will minimally comprise of two layers of inner containment, ice, dri-ice or liquid Nitrogen as appropriate and then two layers of robust outer containment (for example a box formerly used for delivery of radioisotopes). On receipt of such goods into the laboratory, the inner containment layers must all be disposed of as if they were potentially contaminated. Outer layers must be disinfected before disposal or reuse. 23. In event of emergency, priority is to the safety and welfare of staff. If time permits, replace the trypanosomes in sealed containers. Otherwise, dispose of all trypanosomes into ethanol, chloros, trigene or bleach. If in doubt, do the latter.