Electronic supplementary material
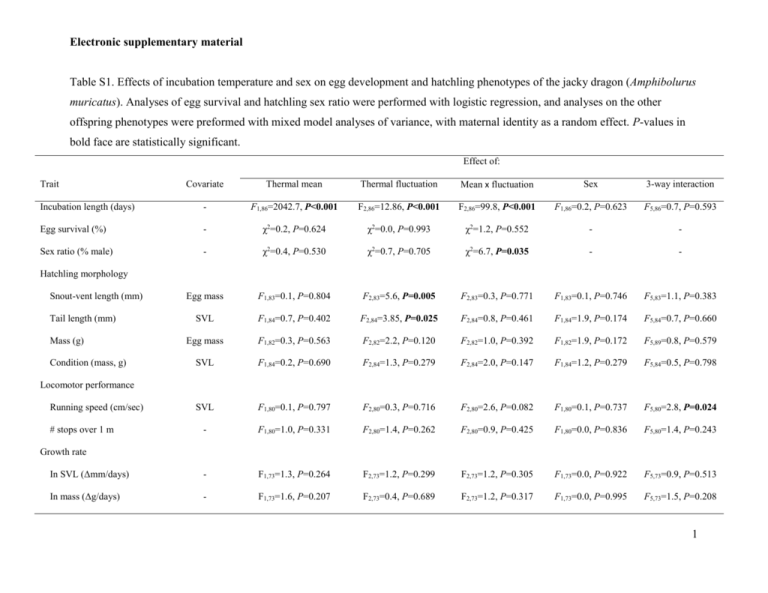
Table S1. Effects of incubation temperature and sex on egg development and hatchling phenotypes of the jacky dragon (Amphibolurus
muricatus). Analyses of egg survival and hatchling sex ratio were performed with logistic regression, and analyses on the other
offspring phenotypes were preformed with mixed model analyses of variance, with maternal identity as a random effect. P-values in
bold face are statistically significant.
Effect of:
Covariate
Thermal mean
Thermal fluctuation
Mean x fluctuation
Sex
3-way interaction
Incubation length (days)
-
F1,86=2042.7, P<0.001
F2,86=12.86, P<0.001
F2,86=99.8, P<0.001
F1,86=0.2, P=0.623
F5,86=0.7, P=0.593
Egg survival (%)
-
χ2=0.2, P=0.624
χ2=0.0, P=0.993
χ2=1.2, P=0.552
-
-
Sex ratio (% male)
-
χ2=0.4, P=0.530
χ2=0.7, P=0.705
χ2=6.7, P=0.035
-
-
Egg mass
F1,83=0.1, P=0.804
F2,83=5.6, P=0.005
F2,83=0.3, P=0.771
F1,83=0.1, P=0.746
F5,83=1.1, P=0.383
SVL
F1,84=0.7, P=0.402
F2,84=3.85, P=0.025
F2,84=0.8, P=0.461
F1,84=1.9, P=0.174
F5,84=0.7, P=0.660
Egg mass
F1,82=0.3, P=0.563
F2,82=2.2, P=0.120
F2,82=1.0, P=0.392
F1,82=1.9, P=0.172
F5,89=0.8, P=0.579
SVL
F1,84=0.2, P=0.690
F2,84=1.3, P=0.279
F2,84=2.0, P=0.147
F1,84=1.2, P=0.279
F5,84=0.5, P=0.798
SVL
F1,80=0.1, P=0.797
F2,80=0.3, P=0.716
F2,80=2.6, P=0.082
F1,80=0.1, P=0.737
F5,80=2.8, P=0.024
-
F1,80=1.0, P=0.331
F2,80=1.4, P=0.262
F2,80=0.9, P=0.425
F1,80=0.0, P=0.836
F5,80=1.4, P=0.243
In SVL (Δmm/days)
-
F1,73=1.3, P=0.264
F2,73=1.2, P=0.299
F2,73=1.2, P=0.305
F1,73=0.0, P=0.922
F5,73=0.9, P=0.513
In mass (Δg/days)
-
F1,73=1.6, P=0.207
F2,73=0.4, P=0.689
F2,73=1.2, P=0.317
F1,73=0.0, P=0.995
F5,73=1.5, P=0.208
Trait
Hatchling morphology
Snout-vent length (mm)
Tail length (mm)
Mass (g)
Condition (mass, g)
Locomotor performance
Running speed (cm/sec)
# stops over 1 m
Growth rate
1
Table S2. Expected and observed sex ratios under six experimental incubation treatments.
Expected sex ratios were derived by calculating constant temperature equivalents (CTE) from the
laboratory incubation treatments and comparing CTE values with results from a previouslypublished constant temperature incubation experiment (Harlow & Taylor 2000). The CTE is a
value derived from thermal regimes that fluctuate around a constant mean and is equivalent to
constant temperature incubation. Calculation of the CTE is based on a developmental zero of
17.2°C (see figure S1); specific details are provided in Georges (1989) and Georges et al. (1994,
2004).
Incubation treatment
CTE
Expected sex ratio (% male) from
Observed sex ratio (% male)
constant incubation at the CTEa
in present study
25°C constant
25.0°C
0
18.1
25±4°C
26.8°C
22.2b
30.0
25±8°C
30.4°C
33.3
50.0
28°C constant
28.0°C
30.0
42.9
28±4°C
29.4°C
40.0
31.6
28±8°C
32.6°C
26.9
21.1
a
Eggs used in the constant incubation experiment (Harlow & Taylor 2000) were from different
study populations than those used in the present study.
b
Sex ratio reported is from a constant 26°C because Harlow & Taylor (2000) did not incubate
eggs at a constant 27°C.
2
100
0.25
(b)
developmental rate
(embryo stages/day)
incubation duration (days)
(a)
90
80
70
60
50
0.20
0.15
0.10
0.05
0.00
40
22
24
26
28
30
32
o
34
incubation temperature ( C)
developmental zero = 17.2oC
18 20 22 24 26 28 30 32 34
incubation temperature (oC)
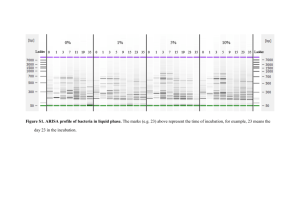
Figure S1. Establishment of the developmental zero (i.e., the temperature at which development
is arrested) for jacky dragon (Amphibolurus muricatus) embryos. The data for incubation
duration at different temperatures (a) were derived from a previously-published study (Harlow &
Taylor 2000). Developmental rate was calculated as the number of embryo stages encompassed
during incubation divided by incubation duration (days) at each temperature. For A. muricatus,
eggs are laid at embryonic stage 31 (Harlow 2004) and hatch at stage 40. Developmental rate
was then regressed against incubation temperature (b). The regression equation (y=0.015x-0.260)
predicted that the temperature at which development is arrested (i.e., zero development) is
17.2°C. This methodology for calculating the developmental zero gives equivalent results to
regressing the inverse of incubation period against temperature (Georges 1989).
3
sex ratio (% male)
100
80
60
40
20
0
24
26
28
30
32
o
CTE ( C)
Figure S2. Relationship between constant temperature equivalent (CTE) and sex ratio in natural
nests of the jacky dragon (Amphibolurus muricatus). Statistics are reported in the text.
4
Figure S3. Effects of incubation temperature means and thermal fluctuations on (a) hatchling
snout-vent length and (b) tail length. Statistics are reported in Table S1. Least-squares means are
reported. Error bars represent 1 SE.
5
REFERENCES
Georges, A. 1989 Female turtles from hot nests: is it duration of incubation or proportion of
development at high temperatures that matter? Oecologia 81, 323-328.
Georges, A., Limpus, C. & Stoutjeskijk, R. 1994 Hatchling sex in the marine turtle Caretta
caretta is determined by proportion of development at a temperature, not daily duration of
exposure. J. Exp. Zool. 270, 432-444.
Georges, A. Doody, S., Beggs, K. & Young, J. 2004 Thermal models of TSD under laboratory
and field conditions. In Temperature-Dependent Sex Determination in Vertebrates (eds N.
Valenzuela & V. A. Lance), pp. 79-89. Washington DC: Smithsonian Institution Press.
Harlow, P. S. 2004 Temperature-dependent sex determination in lizards. In TemperatureDependent Sex Determination in Vertebrates (eds N. Valenzuela & V. A. Lance), pp. 42-52.
Washington DC: Smithsonian Institution Press.
Harlow, P. S. & Taylor, J. E. 2000 Reproductive ecology of the jacky dragon (Amphibolurus
muricatus): an agamid lizard with temperature-dependent sex determination. Aust. Ecol. 25,
640-652.
6