Clausius-Clapeyron Equation
advertisement
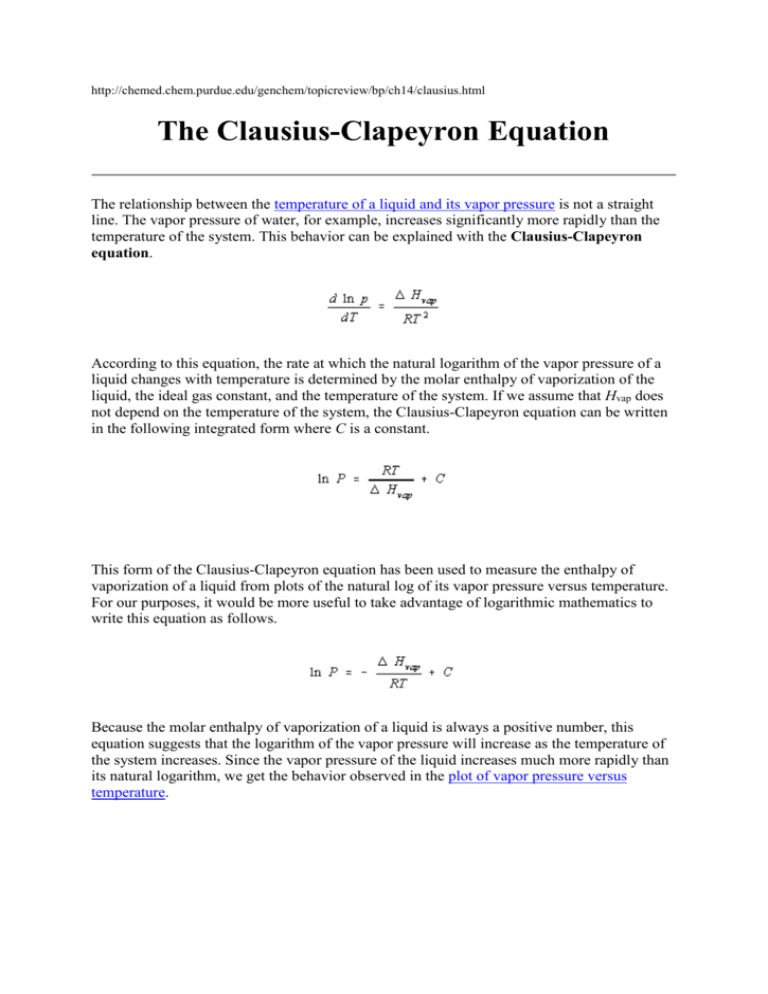
http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch14/clausius.html The Clausius-Clapeyron Equation The relationship between the temperature of a liquid and its vapor pressure is not a straight line. The vapor pressure of water, for example, increases significantly more rapidly than the temperature of the system. This behavior can be explained with the Clausius-Clapeyron equation. According to this equation, the rate at which the natural logarithm of the vapor pressure of a liquid changes with temperature is determined by the molar enthalpy of vaporization of the liquid, the ideal gas constant, and the temperature of the system. If we assume that Hvap does not depend on the temperature of the system, the Clausius-Clapeyron equation can be written in the following integrated form where C is a constant. This form of the Clausius-Clapeyron equation has been used to measure the enthalpy of vaporization of a liquid from plots of the natural log of its vapor pressure versus temperature. For our purposes, it would be more useful to take advantage of logarithmic mathematics to write this equation as follows. Because the molar enthalpy of vaporization of a liquid is always a positive number, this equation suggests that the logarithm of the vapor pressure will increase as the temperature of the system increases. Since the vapor pressure of the liquid increases much more rapidly than its natural logarithm, we get the behavior observed in the plot of vapor pressure versus temperature. http://learn.chem.vt.edu/tutorials/lsproperties/clauclap.html Liquid-Vapor equilibrium Clausius-Clapeyron Equation The vapor pressure of a liquid increases as the temperature increases. This is why puddles left after a rainstorm evaporate faster on a hot day than on a cold day. The vapor pressure of a liquid increases faster as the temperature nears the boiling point of the liquid- the data is not a straight line. However, it turns out that the plot of the log of the vapor pressure vs. 1/T is a straight line with a slope equal to that of -Hvap/R. We can use this fact to derive a simple equation that relates the vapor pressure at certain temperatures to the heat of vaporization, the Clausius-Clapeyron Equation. The equation for a straight line is y=mx+b, where m is the slope and b is the y-intercept. For our case, the y values are ln(Pvap) and the x values 1/T and the slope m = -Hvap/R. Thus, the equation for the line is ln(Pvap) = -Hvap/RT + b If we discover the vapor pressure at two separate temperatures, we have two points on the same line. 1. ln(P2) = -Hvap/RT2 + b 2. ln(P1) = -Hvap/RT1 + b If we subtract equation 1 from equation 2, the b's cancel and we're left with ln(P2) - ln(P1) = -Hvap/R * (1/T2- 1/T1) or ln(P2/P1) = -Hvap/R * (1/T2- 1/T1) which is known as the Clausius-Clapeyron Equation. This equation will let us figure out the vapor pressure of a liquid at any temperature if we know the heat of vaporization, or find out the heat of vaporization if we know the vapor pressure at two different temperatures. Example 1: Water has a vapor pressure of 24 mmHg at 25oC and a heat of vaporization of 40.7 kJ/mol. What is the vapor pressure of water at 67oC? Solution: Simply use the Clausius-Clapeyron Equation to figure out the vapor pressure. We have to be a bit careful about the units of R: the units we're using are kJ, so R = 8.31x10-3 kJ/mol K. ln(P2/P1) = -Hvap/R * (1/T2- 1/T1) ln(P2/24) = 40.7 kJ/8.31x10-3 kJ/mol K *(1/340- 1/298) ln(P2/24) = 2.03 P2/24 = 7.62 P2 = 182 mmHg Example 2: An unknown liquid has a vapor pressure of 88mmHg at 45oC and 39 mmHg at 25oC. What is its heat of vaporization? Solution: Again, use the Clausius-Clapeyron Equation. Here, the only thing we don't know is Hvap ln(P2/P1) = -Hvap/R * (1/T2- 1/T1) ln(88/39) = -Hvap/8.31x10-3*(1/318 - 1/298) Hvap = 32.0 kJ Back to the liquids and solids index page Výpočet pre DMC (dimetylkarbonát) p1 = 1 atm pri b.p. = T1 = 90°C p2 = ? pri T2 = 210°C výparná entalpia deltaH = 37.7kJ/mol (Beilstein) ln(p2/1)= 37.7(1/363-1/483)/8.314*10-3=3.104 p2 = 22.3atm pri 210°C Výpočet pre Ac2O p1 = 1 atm pri b.p. = T1 = 139 °C (412) p2 = ? pri T2 = 200°C (473 K) výparná entalpia deltaH = 52 kJ/mol (Beilstein) ln(p2/1)= 52 (1/421-1/473)/8.314*10-3=1.633 p2 = 5.12 atm pri 200°C Výpočet pre tBuOH p1 = 1 atm pri b.p. = T1 = 82.15°C (355) p2 = ? pri T2 = 150°C (423) výparná entalpia deltaH = 45.6kJ/mol (Beilstein) ln(p2/1)= 45.6(1/355-1/423)/8.314*10-3 = 2.484 p2 = 12 atm pri 150°C Výpočet pre EtOH (14.2 KJ/mol 230°C, 35.2 pri 120°C, 42.7 pri 25°C) p1 = 1 atm pri b.p. = T1 = 78°C (351) p2 = ? pri T2 = 150°C (423) výparná entalpia deltaH = 42.6kJ/mol (Beilstein) ln(p2/1)= 42.6(1/351-1/423)/8.314*10-3 = 2.48 p2 = 12 atm pri 150°C











