Trizol RNA Isolation from Cultured Cells or Tissues
advertisement

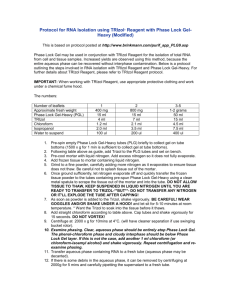
Trizol RNA Isolation from Cultured Cells or Tissues Solutions: Trizol cat # 15596 GibcoBRL 2 M Sodium Acetate Add 16.42 g of sodium acetate (anhydrous) to 40 ml H2O and 35 ml of glacial acetic acid. Adjust solution to pH 4 with glacial acetic acid and the final volume to 100 ml with H2O. The solution is 2 M with respect to the sodium ions. Phenol Chloroform/isoamyl alcohol Isopropanol 75% EtOH Note: Carry all steps out at room temperature unless otherwise noted. 1a. For tissue, add 1 ml Trizol/100 mg tissue and homogenize with a few strokes in a glass Teflon homogenizer. 1b. For cultured cells, either centrifuge suspension cells and discard supernatant, or remove the culture medium from the cells grown in monolayer cultures. Add 1 ml Trizol/107 cells (10 cm dish) and pass the lysate through a pipet seven to ten times. Transfer to microcentrifuge tube. -do not wash cells with saline. Cells grown in monolayer can be lysed directly in the culture dish or flask. 2. Incubate the homogenized sample for 5 minutes at RT to permit the complete dissociation of nucleoprotein complexes. Add 0.2 ml of chloroform per 1 ml of Trizol. Shake vigorously for 15 seconds and incubate at RT for 2-3 minutes. 3. Centrifuge 15 min, 12,000xg at 4 C. Transfer the upper aqueous phase to a fresh tube. -the upper aqueous phase contains the RNA, whereas the DNA and proteins are in the interphase and lower phenol/chloroform phase. The volume of the aqueous phase is ~1 ml, equal to the initial volume of Trizol. 4. Precipitate the RNA by adding 0.5 ml (per 1 ml of Trizol) of 100% isopropanol. Incubate samples for 10 minutes at RT. Centrifuge 10 minutes at 12,000xg, 4 C, and discard supernatent. 5. Wash RNA pellet once with 1 ml 75% ethanol, vortex and centrifuge 12,000xg for 5 min at 4 C. 6. Let pellet briefly dry and dissolve the RNA pellet in 100 to 200 µl DEPC-treated H2O or in DEPC-treated 0.5% SDS, depending on the subsequent use of RNA. Store samples frozen at 70 C or in ethanol at -20 C.