P366ASyllabusDunning - Sonoma State University
advertisement

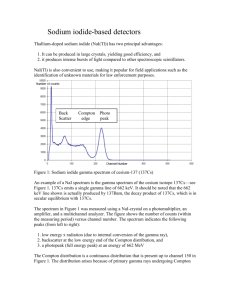
Sonoma State University Spring 2006 Physics 366A John Dunning Laboratory Outline Gamma Ray Detection and X-ray Diffraction The first four experiments are an introduction to neutron activation analysis using our Intrinsic Germanium Detector to analyze gamma rays, uranium detection in luggage and particulate air sampling. The next three experiments introduce X-ray diffraction as an analytic tool for determining crystal structure and compound identification using Jade for search/match. Text: Introduction to Nuclear Engineering 3rd edition (2001) by Lamarsh and Baretta, Prentice Hall. Chart of the Nuclides 16th edition (2002) available to students for $15 from Lockheed Martin. The instructor has purchased 10 copies in advance for the class. Extra student copies can be purchased following instructions at http://www.chartofthenuclides.com/ Eight lectures are planned. Six are on Mondays starting January 30th; two are on the Wednesday when new instrumentation is started: February 1 st and March 1st. The ninth lecture is an open book quiz. The groups starting Nuclear should take these lectures and the quiz. Friday’s 10:30 AM – 3 PM will be extra access time to the equipment we are using and this can accommodate additional groups. Dr. Shi will conduct separate lectures covering SEM/EDX, Auger Spectroscopy, AFM and its applications and sample preparation by vacuum deposition. To provide good access to the equipment, if there are sufficient students, we will split the class and both sets of lectures will be given simultaneously. Then the groups will switch and the experiments offered anew. The grade for this part of the course is based on: Quiz 20%, Homework 20%, Experiments graded in the laboratory book 60%. Ruled Laboratory notebooks are required to record all your work, including all the procedures, data, analysis, etc. The sequences of experiments below are to guide you through initially unfamiliar instrumentation. Proceed at a pace that optimally enhances your understanding of both the physics and the instrumentation. Record this progress in the laboratory book in sufficient detail that you could repeat the experiment two years from now if your job were such that you needed to work with this equipment. Gamma detection: all in Carignane. Four sessions total all start on Wednesday We have a fine, high resolution, High Purity Germanium (HPGe) gamma detector, associated digital processing hardware and Genie 2000 analysis program which has flexible libraries. Familiarization Exercise (first Wednesday at 1 PM): Use a Geiger counter to determine the activity and penetrating power of our beta/gamma calibration sources. Radiation Safety with small sources. First Session (later the same Wednesday): Introduction to gamma spectroscopy. Neutron activation analysis of human hair and other samples. These samples were activated in the Washington State University Reactor in fall of 2002. They were counted at SSU a few days later for longer half life gammas. Each group can pick a different spectrum and determine the elements present. Both nuclear lab computers have all spectra loaded so two groups can work simultaneously. Second Session: All groups do the second gamma experiment at different times. This experiment aims to familiarize you with gamma calibration sources and to energy calibrate our detector. After calibration you can study the thorium series gammas using a gasoline lantern mantle as a source. Third session: The Hot Luggage Laboratory. This laboratory has you measure the gamma/X-ray spectrum of natural uranium and depleted uranium with and without shielding. From these measurements you will be better able to design an appropriate detection scheme. Fourth session: The hot air laboratory. Obtain a two hour sample of the particulates in room air using a filter paper and a high volume air sampler. Measure the gamma spectrums of the short half lived emitters found and determine their origin. XRD introduction: Three sessions all in the Keck laboratory, Salazar 2009B. First Session. Two exercises using interesting demonstration spectra help you to acquire initial familiarity with the powder diffraction file and the Jade analysis software. I hope to have Jade 7.5 – the latest version – up and running. Second Session: Sample preparation. Under supervision, each group grinds, runs and analyzes a one or two phase unknown using reagents from the chemistry stockroom. Third session: Safety/machine protocol quiz. You are on your way to becoming an operator. Run two suitably complex unknowns of different character. One should be a mineral. The other can be an interesting stamp or a metal. Nuclear and X-ray Lectures Below is my planned topic sequence. Handouts in addition to our two texts are in the works. Interesting problems will be offered with solutions given one week after the topic is discussed in class. These lectures will be repeated if the course is divided into two groups. The laboratory lectures are instrument specific. 1) Monday January 30: Chart of the Nuclides, beta decay, gamma deexcitation, radioactive decay, start neutron activation analysis. Lamarsh section 2.8 Equation 2-30 describes neutron activation and the saturation factor, Chart of Nuclides. 2) Laboratory Lecture Wednesday Feb 1st. Beta/gamma familiarization experiment using a Geiger counter ½ hour. Neutron activation continued, the saturation factor, gamma ray interactions in our detector. Introduction to Genie 2000 analysis routines in use. Break. Laboratory. Start software introduction laboratory. We have two computers loaded with Genie 2000 and neutron activation spectrum to choose from. 3) Monday February 6th: Alpha decay and Uranium-238, 235 decay series, secular equilibrium. The age of the Alliende meteorite using U/Pb ratio method. Handout on age of the earth. Gamma calibration experiments start Wednesday February 8th. Groups schedule for 2 hr blocks of time with the Ge detector on Wednesday or Friday afternoons as needed. 4) Monday February 13th : Interaction of gamma rays with matter, exponential attenuation in matter, mass attenuation coefficients, mass-energy absorption coefficients. Lamarsh section 3.8 Point source gamma shielding calculations using build up factors. Lamarsh section 10.1. 5) Monday February 27th: Discussion of shielding homework problem. Introduction to X-ray Diffraction. The Bragg equation. The powder method. Cullity p 1-19 Handout. 6) Laboratory Lecture Wednesday March 1st: X-ray safety. Handout + video. Introduction to Jade 6.5 and the ICDD data base. Search/match laboratory with each group scheduled for a 2 hour block. 7) Monday March 6th: X-ray production: Energy vs. wavelength. Fraction of energy in bremsstrahlung, Characteristic X-rays, our monochromator. Comparison to synchrotron radiation sources. 8) Monday March 13th: Absorption of X-rays in our powder samples. What constitutes an infinitely thick target? Target thickness vs. angle. Discussion of homework problems. Cullity p. 152-154 handout . Wednesday March 15th the third and final XRD lab starts. 9) Monday March 20th quiz. March 22nd. Groups switch between Shi and Dunning on Wednesday Useful internet references: X-ray emission energies http://xdb.lbl.gov/Section1/Sec_1-2.html These also work for the EDX mounted on the SEM Mass attenuation coefficients: http://physics.nist.gov/PhysRefData/XrayMassCoef/tab3.html Stopping power (1/density dE/dx), and Range tables: http://physics.nist.gov/PhysRefData/Star/Text/contents.html The electron stopping power is useful for understanding the SEM. Problem Suggestions #1 Due Monday February 13th. Two more sets will be handed out. One on gamma ray shielding, the other on X-ray diffraction and target thickness. Solutions given on the due date. Use the Chart of the Nuclides for nuclear data. 1. P-32 is used in many biological research projects because it can be used to tag DNA fragments. What is detected? 2. I-131 is used to test the uptake of the thyroid. What types of radiation and energy are emitted? What is actually detected outside the patient? 3. Compare the Chart of the nuclides listing for Co-60 with the decay scheme on p. 22 of our text. Are the two gamma rays always emitted in coincidence (one rapidly following the other from the same decay), or does one decay emit one gamma and another decay emits the second gamma? These gammas are used to calibrate our Germanium detector. 4. Co-57 decays by electron capture. What is the product isotope? What is the most abundant gamma, the second most abundant gamma? These gammas of these will be used to calibrate our Germanium detector. In addition there are K X-rays from the product atom. These are listed in red on the periodic table of the elements in the back of the Chart of the Nuclides. What are the energies of these two X-rays? 5. For U-238, consider the first three members of the decay series. List the dominant decay mode and energy of the emitted particle. For beta particles this will be the end point energy. 6. Lamarsh: problem 2-29 7. Lamarsh: problem 2-37 This 3% (by weight) enrichment of uranium is used in many nuclear power plants. The abundances on the chart of the nuclides are atom %. Assume only the U-235 is radioactive for a start, and then upgrade the estimate. 8. a) You desire to detect aluminum using neutron activation. Write down the reaction used and the gamma ray energy detected. b) How long of an exposure should you undertake to obtain 3/4 of the maximum possible aluminum activity? c) The gamma rays from sodium neutron activation decay products have different energies, but they can be sufficiently numerous to cause problems. Sodium occurs in many samples. Including the samples used in the introductory experiment. Write down the neutron activation reaction and the gamma ray energies expected. What fraction of the maximum possible activity of the appropriate sodium isotope do you have using the exposure time in part "b"? d) If you wished to minimize the sodium background, would a longer exposure be advisable in these circumstances?