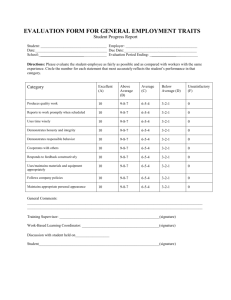
rinse flush
advertisement

Program-wide Scope Reprocessing Competency Package NOTE: THIS DOCUMENT APPLIES TO any department where flexible endoscopes are used and reprocessed This document was developed by Kaiser Permanente. Please feel free to use and reproduce these materials in the spirit of patient safety, and please retain this note in the spirit of appropriate recognition. Kaiser Foundation Hospitals Originally Created by: National KP Disinfection and Sterilization Task Force 2001 (Last revised by NCal KP Scope reprocessing experts: December 2014) Table of Contents 1. Overview/CEU Credit Page 3 2. Position Statement/References Page 4 3. Behavioral Goal/Objectives Page 5 4. Scope Reprocessing Policy Page 6-7 5. Scope Reprocessing Procedure Page 8-12 6. Scope Reprocessing Quick Reference Grid Page 13 7. APIC Endoscope Reprocessing Guidelines Page 14 8. 5SGNA Endoscope Reprocessing Guidelines Page 15 9. Hand hygiene Page 16 10. Point-of-use precleaning Page 17-18 11. Leak testing Page 19-20 12. Manual cleaning Page 21-22 13. Testing MEC of HLD and Activation/Deactivation Page 23-24 14. Manual HLD Page 25-26 15. Custom Ultrasonic - DOS Operating System Page 27-28 16. Custom Ultrasonic - Windows Operating System Page 29-30 17. Medivators Automated HLD Page 31-32 18. Scope Storage Page 33-34 19. Documentation and Record keeping Page 35 20. Sterrad NX Page 36-37 21. Chemical spill response Page 38 22. Disinfectants: Glutaraldehyde Ortho-phthalaldehyde Resert XL Page 39-40 Page 41-42 Page 43-44 23. Audit Tool example Page 45-46 24. Post Test Page 47-48 25. Post Test Answer Key (for manager access only) Page 49 26. Documentation of Employee Completion of Competency Package Page 50 27. Definitions Page 51 28. Algorithm: Investigation for potential exposure related to Scope Reprocessing Page 52 29. Sample Scope Training Log Page 53 2 1. Overview In order to ensure competency in scope reprocessing, each staff person should accomplish the following steps. This should be done at the time of hire and annually thereafter to demonstrate competency according to the 1999 FDA Recommendations: 1. Staff is responsible to review Scope Reprocessing Program Policy and Procedure and Quick Reference Grid (attached). 2. Managers are responsible to review the APIC Guidelines for Infection Prevention and Control in Flexible Endoscopy; American Journal of Infection Control, 1999; 28:138-155. Copies can be downloaded from the following website: http://www.apic.org/AM/Template.cfm?Section=Guidelines&CONTENTID=6381&TEMPLATE=/CM/ContentDisplay.cf m). This should be available for staff to reference. 3. Staff is responsible to review the following SGNA scope reprocessing guidelines “Steps Necessary to Thoroughly Clean and High Level Disinfect Flexible Endoscopes”; Society of Gastroenterology Nurses and Associates, Inc., Edition 2012. Website: http://www.sgna.org. Additional scope specific information for Olympus and Pentax scopes available at: http://www.olympusamerica.com/msg_section/index.asp, http://www.pentaxmedical.com/ and http://www.fujinonendoscopy.com/default.aspx?pageid=218 4. Staff is responsible to watch the appropriate scope videos – i.e., http://www.olympusamerica.com/msg_section/cds/cds_resources.asp 5. Staff is required to provide return demonstration for all reprocessing tasks including: Completing Competency Tool with observer/trainer and successfully perform all reprocessing tasks in compliance with Medical Center Policies and Procedures, including: PPE: Personal Protective equipment Point of Use Pre-cleaning Leak Testing Manual Cleaning Disinfection/Sterilization Rinsing/Drying Accessories Storage Appropriate Preventative Maintenance and Record Keeping 6. Staff must complete documentation of competency by: Completing post test; reviewing answers with observer/trainer; completing Documentation Form included in this package. NOTE ON CEU CREDIT: Continuing education credit should be available at the local level for any employee who completes this competency package. Contact your local training/education staff or department manager. NOTE: For information regarding fees and scheduling of intensive training classes on Custom Ultrasonics machine use, contact CU staff at 215-364-1477. For information on Custom Ultrasonics Training by Kaiser Permanente, contact: Teresa McLaren at: 510-559-5129 or teresa.mclaren@kp.org 3 2. Position Statement The Kaiser Permanente Interregional Disinfection and Sterilization Work Group supports a national standardized approach to scope reprocessing in an effort to ensure appropriate, safe and effective reprocessing of this equipment. The attached policy, procedure, quick reference grid and competency tools were developed to this end. These documents were written in accordance with the references listed below, and were designed to be utilized as standard reference guidelines for the Kaiser Permanente organization. These attached documents should be placed in manuals for staff reference in departments where scopes are utilized and reprocessed. A copy of these documents will also be referenced in the standardized Infection Prevention and Control Ambulatory Care Manual, which was distributed nationally in 1998, and is updated every 2 years. REFERENCES: Alvarado, C.J., Reichelder, M., "APIC Guideline for Infection Prevention and Control in Flexible Endoscopy"; American Journal of Infection Control (AJIC). April 2000, Vol. 28, No.2. American Society for Gastrointestinal Endoscopy. Infection Control During GI Endoscopy Guidelines. Ww.giejournal.org Volume 67, No.6:2008 Association for the Advancement of Medical Instrumentation, Chemical sterilization and high level disinfection in health care facilities ANSI/AAMI ST 58:2013: www.aami.org Catalone, Bradley, Drosnock, Mary Ann. “Storing endoscopes: How long is too long?” Health Purchasing News (HPN). November 2010. Print. CDC Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Disinfection_Nov_2008.pdf CDC Guidelines for Environmental Infection Control in Health-Care Facilities http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/Enviro_guide_03.pdf Davis DA, Zadinsky JR, Carrel C, "Flexible Endoscopy: Compliance Training Program", Infection Control and Sterilization Technology (ICST), September 1998, pp. 24-34. FDA and CDC Public Health Advisory: Infections from Endoscopes Inadequately Reprocessed by an Automated Endoscope Reprocessing System, September 10, 1999. FDA, CDC and VA Safety Communication: Preventing Cross-contamination in Endoscope Reprocessing. November 19, 2009. http://www.fda.gov/mMedicalDevices/Safety/Alertsand Notices/ucm190273.htm FDA Alert: Liquid Chemical Germicide Residue Causes Burns, August 26, 2002 International Association of Healthcare Central Service Materials Management: http://iahcsmm.org/ Medivators. http://www.medivators.com/customer-support/procedure-product-ifus Petersen, B et al, “ASGE and SHEA guideline - Multisociety Guideline on Reprocessing Flexible GI Endoscopes”; Infection Control and Hospital Epidemiology (ICHE). June 2011, Vol. 32, No. 6. Occupational Safety and Health Administration. "Occupational Exposure to Bloodborne Pathogens Final Rule", 29 C.F.R., Part 1910.1030, Federal Register (December 6, 1991). Olympus. http://www.olympusamerica.com/msg_section/cds/cds_position.asp PCI Medical. http://www.pcimedical.com/acc/acc2.htm Spaulding EH. "Chemical Disinfection of Medical and Surgical Materials", Disinfection, Sterilization and Preservation, Philadelphia, PA: Lea & Febiger, 1968. Society of Gastroenterology Nurses and Associates, Inc. "Standards for Infection Control and Reprocessing of Flexible Gastrointestinal Endoscopes", 2009: www.SGNA.org 4 3. Behavioral Goals and Objectives Goals: 1. Ensure that all employees who handle scopes comply with Kaiser policies, manufacturer’s instructions and professional organization guidelines for cleaning and disinfection or sterilization of scopes by the manual or automatic reprocessor or sterilization method as outlined by the procedures following. 2. Ensure consolidation of scope reprocessing processes wherever possible, in order to improve patient and employee outcomes. Consolidation of reprocessing improves outcomes by minimizing employee exposure to chemical disinfectants and by ensuring that reprocessing is performed by a core group of fully trained employees only. Objectives: After completion of the Scope Reprocessing Competency Package, the employee will be able to provide return demonstration for all reprocessing tasks including: Perform hand hygiene per protocol. Perform all reprocessing activities using appropriate personal protective equipment. Perform point of Use Pre-cleaning prior to transporting scope to reprocessing area. Perform leak testing either manually, automated or computerized. Correctly disassemble and clean scopes as described in the procedure. Perform proper cleaning and high level disinfection or sterilization procedures appropriate to the scope being processed. Perform proper drying procedures using alcohol and forced air appropriate to the scope being reprocess. Demonstrate understanding of the process monitors (i.e. test strips, monitoring tapes, records, etc). Demonstrate understanding of required preventative maintenance on automated reprocessing machine if used. Demonstrate correct storage of scopes after appropriate cleaning and maintenance of mandated records. Describe the algorithm for investigating of potential communicable disease exposure (e.g. Hepatitis B, C, Clostridium difficile) related to scope reprocessing. 5 4. Policy 1. Scopes that pass through normally sterile tissue should be subjected to a sterilization procedure before each use; if this is not feasible, they should receive at least high-level disinfection. 2. Scopes that come in contact with mucous membranes are classified as semi-critical items and should receive at least high-level disinfection. 3. Scopes should be repeat reprocessed prior to use if stored for ≥ 30 days between uses (unless sterilized). Ensure a 30 day tracking system is in place. 4. Engineering controls, such as snorkel exhaust or slot exhaust ventilation, should be operating in scope reprocessing areas. 5. Personal protective equipment should be used to protect workers from exposure to infectious agents (e.g. HIV, Hepatitis B virus, Hepatitis C virus, M. tuberculosis, etc.) and chemicals. 6. Reprocessing of scopes should begin with Point of Use Pre-cleaning. Pre-cleaning should be done prior to transporting the scope to the reprocessing area. Pre-cleaning should include wiping the exterior and suctioning all channels of the scope with an approved detergent formulated for endoscopes following the manufacturer recommendations. 7. Follow manufacturer dilution instructions for endoscopes compatible detergent. Care should be taken when diluting the solution to avoid splashing or aerosolization of the product. The scope should always be submerged when flushing with detergent to prevent aerosolization. 8. Scope should be transported to the reprocessing area in an appropriately labeled and covered container. 9. Leak testing is required on some endoscopes (e.g. Pentax) before immersion. For cystoscopes follow according to manufacturer recommendation whether or not to do leak testing. 10. Cleaning of the scope should include flushing and brushing of all channels with an endoscope compatible detergent (e.g. Tergal) for scope cleaning prior to disinfection or sterilization, to remove particulate matter. The scope should always be submerged when flushing to prevent spraying detergent into the air. Irrigation adapters, where applicable, should be used to facilitate cleaning of all channels. All parts of the scope should be rinsed with tap water. Detergents must be discarded after each scope. 11. Cleaning brushes should be disposable or, if reusable, they must be thoroughly cleaned and receive high-level disinfection or sterilization after each use. Cleaning brushes should be examined to ensure they are intact after use and piece(s) have not broken off inside scope. 12. As an alternative to manual flushing during cleaning prior to disinfection, meticulous automated flushing may be accomplished via use of a “pre-processing sink” or endo-flushing device using an endoscope compatible detergent (e.g. Tergal) for scope cleaning prior to disinfection or sterilization. This should be followed by manual brushing and then an ultrasonic “cleaning” cycle in an automated scope reprocessor (AER) machine (e.g. Custom Ultrasonics). Ensure that rinsing after cleaning and proper dilution of detergent is accomplished manually, if not with an automated process. Manual or automated cleaning should be done following the manufacturer and KP procedures. 13. Establish a process for identifying the ERCP scope elevator wire guide channel or the Colonoscope and Gastroscope auxiliary water channel in order to ensure cleaning/disinfection of the extra channel. 14. After cleaning, scopes must be high-level disinfected at a minimum. 15. All internal and external surfaces should be in contact with the disinfectant for not less than the time indicated by the CDC, AORN, AAMI, APIC, SGNA, SHEA and Kaiser Policy (e.g. 20 minutes for 2% glutaraldehyde). 16. Run a wash only diagnostic cycle on the AER to ensure flow through all channels as the first cycle of the day. 17. Don clean PPE before removing scopes from the disinfectant solution or the completion of the AER cycle. 18. Following manual chemical disinfection, scopes must be thoroughly rinsed with copious amounts of sterile/tap water exterior of scope plus 100 mL each lumen/each rinse. For manual disinfection use three water rinses - if using a container, discard water after each rinse. For automated disinfection use two rinses with the minimum default cycle time (e.g. Olympus= three minutes, CU = two and a half minutes). Cidex OPA requires 3 rinses. Only certified AER technicians has the authority to set cycle parameters. 19. All water rinses should be followed by a 5 ml 70% ethyl or isopropyl alcohol flush per lumen, and then followed by drying the scope and all of its channels using forced air follow manufacturer guidelines for introducing the alcohol and air into the channels. For automated endoscope reprocessors finish with alcohol flush and air purge. Visually check that all ports have flow during alcohol purge. If no flow is noted then the AER must be inspected (by facility engineering, third party contractors or Clinical Technology). 20. Channeled scopes must be hung in a vertical position with the distal tip hanging freely in a well-ventilated scope cabinet. Do not attach removable parts (valves, etc.) to further facilitate drying. Remove water resistant cap. 21. Scope accessories that penetrate mucosal barriers (e.g., biopsy forceps) if reusable must be cleaned and sterilized between each patient use. Discard if single use. 22. Sterile water should be used to fill the water bottle (used for cleaning the lens and irrigation during the procedure). If not using disposable endo-flushing pump accessories, e.g. water bottles, cap and tubing, (it is highly recommended to 6 switch from disposable tubing to SmartCap and Endo Gator by Medivators. High-level disinfect or sterilize the water bottle and its connecting tube at least daily (every time following an ERCP procedure). 23. If not using a pre-processing sink which automates flushing of lumens, consider using the EndoFlush system from PCI Medical ( Olympus scopes Only), or the Scope buddy from Medivator to prevent repetitive motion injury 24. Filter changes and routine maintenance should be accomplished and documented on all automated endoscope reprocessors. Reprocessing technicians are responsible for changing inline disk filters every 6 -8 weeks or sooner if needed. Local Facility Engineers, third party contractors or Biomedical Engineers are responsible for changing all other filters and additional preventative maintenance on automated reprocessors. Instructions applicable to specific scope models in use should be made available to all staff responsible for reprocessing. See preventative maintenance section. 25. A log must be maintained including MRN, patient name, MD, date, ID# of scope, procedure type, scope type, and AER # and cycle used. 26. Notify local Infection Prevention and Control and Risk Management if reprocessing deficiencies are identified. 27. A system should be in place to clearly identify which scopes have been reprocessed, and which still need reprocessing. 28. For Sterrad NX, use advanced cycle only for all NX compatible single channeled flexible scopes no longer than 850cm in length and no smaller than 1mm in diameter. NOTE: Sterilized scopes should be processed on a wash only cycle, in the CU AER if approved by the scope manufacturer, and omit alcohol flush. 7 5. Procedure NOTE: Instructions applicable to specific scope models in use should be made available to all staff responsible for reprocessing. 1. Point of Use Pre-cleaning (Procedure Room) NOTE: pre-cleaning is a critical step in ensuring appropriate HLD/ sterilization. Any body fluids or debris left on the instrument may prevent HLD/sterilization. a) Perform hand hygiene per protocol b) Wear PPE during all reprocessing steps, the following PPE is required in the procedure room -impervious gown, nitrile gloves and surgical mask with splash guard. c) Follow manufacturer dilution instructions for endoscope compatible detergent solution. Care should be taken when diluting the solution to avoid splashing or aerosolization of the product. d) Immediately after removing the scope from the patient, turn off video processor and light source, and then gently wipe the insertion tube from the control section to the distal tip with a “lint-free cloth” or sponge soaked with freshly prepared endoscope compatible detergent. Turn on suction pump. (NOTE: lint-free cloth or sponge should be discarded after use). e) Immerse the distal end of the scope into endoscope compatible detergent solution and aspirate for 30 seconds. Remove the tip from the detergent and aspirate air for 10 seconds. f) Turn off the suction pump. Flush water and air into the air/water channel. With the light source off, remove the air/water valve. Insert the air/water channel cleaning adapter. g) Turn on the light source and switch air flow regulator to maximum output. Immerse the distal end of the scope into endoscope detergent compatible container and depress the air/water channel cleaning adapter valve for 30 seconds. Release the valve to feed air through the air channel for 10 seconds. h) If the scope has auxiliary water channel whether it was used or not, connect auxiliary tube to the auxiliary channel and using a 30ml syringe flush detergent through channel. Following by flush water and air. NOTE: If reprocessing a duodenoscope, regardless of use, connect washing tube to elevator wire guide channel plug and using a 5ml syringe flush detergent through channel. Follow by flushing with water and air Follow the manufacturer instruction. i) Turn off the light source. Remove all valves and place in compatible detergent. Disconnect suction tube, water container and video scope connector. j) Detach the scope from the light source and suction pump. Attach protective water resistant video cap (if using video endoscope). k) Transport scope in an appropriately labeled covered container to reprocessing area. Leakage Test (Prior to Manual or Automated Disinfection) a) Perform hand hygiene per protocol. b) Wear PPE during all reprocessing steps, include a minimum, Nitrile gloves, an impervious gown, hair covering, mask and eye protection such as safety chemical goggles during scope reprocessing c) Visibly inspect the scope for holes, tears, and other damage after each patient use. d) Ensure that all valves have been removed (i.e. suction, air/water and biopsy valves) e) Tighten light guide prong prior to immersion. f) Leak test the scope after each patient use according to manufacturer instructions. g) Prior to immersing scope in any fluid visually inspect the water resistant cap for damage. If it is damaged or wet, use another cap. h) Attach water resistant cap to scope. (Never connect or disconnect water resistant cap while the scope or cap is immersed as it will damage the scope. i) Attach the leakage tester to the air source and confirm that air is being emitted. j) Attach the leakage tester to the scope and confirmed that the bending section inflation. k) Immerse the scope in clean water. DO NOT leak test in detergent as it hinders visualization of leaks. l) With the angulation locks in the free position, angulated the distal tip of the scope in all directions observing for bubbles. m) Observed control knob, control buttons, insertion tube, distal bending section, all channels, vale ports, connectors and universal cord for bubbles coming from the interior of the scope. n) If no leak is observed, remove the scope from the water, and turn off air supply. i. Manual: press the pressure release valve to fully deflate scope prior to disconnecting leak tester from scope. The process is complete. ii. Automatic: turn off machine, disconnect the leakage tester from the air supply and wait (20-25 seconds) for the bending section rubber to return to normal size (depressurize). Detach the leakage tester from the water resistant cap. The process is complete. 8 o) When a leak is detected, (e.g., continuous bubbles coming from the scope), follow specific scope manufacturer processing procedure before sending for repair. E.g. Olympus recommends disinfection prior to shipping. The leak tester must remain attached during this process. A scope sent in for repair should be considered a contaminated medical device and labeled as such for shipping. Labeling should be accomplished with a biohazard label and supplemental label. The supplemental label must indicate which portions of the scopes remain contaminated. Cleaning a) Perform hand hygiene per protocol. b) Wear PPE during all reprocessing steps; include a minimum, nitrile gloves, an impervious gown, hair covering, mask and eye protection such as safety chemical goggles during scope reprocessing c) Prepare a sink or basin with freshly-made solution of water and endoscope compatible detergent, diluting and using according to the detergent manufacturer’s instructions. NOTE: fresh endoscope compatible detergent must be used for each scope to prevent cross-contamination. d) Remove and soak all removable parts of the scope in an endoscope compatible detergent and clean these parts using a brush. e) Carefully coil the scope and completely immerse in compatible detergent. f) Use a soft brush or lint free cloth or sponge to clean the exterior surface of the scope. Pay close attention to the distal tip to ensure that the air/water nozzle is free of debris. Take care not to scratch the small lenses. g) Perform all brushing while submerged to prevent splashing and aerosolization of contaminated fluid. Duodenoscope (ERCP), and Colonoscope, Gastroscope reprocessing: h) Establish a process for identifying the ERCP scope in order to ensure cleaning/disinfection of the elevator wire guide channel. i) Raise the elevator on ERCP scope and carefully brush around and under the elevator mechanism. Lower elevator before brushing the channels. j) Brush the channels a minimum of 3 times per channel. Continue until there is no visible debris. Start by inspecting the channel cleaning brush for any damage. There are 2 portions of the suction channel that require individual brushing. i. Extends from the valve housing to the distal tip ii. Extends from the valve housing to the light source connector k) Brush suction channel within the insertion tube by holding channel cleaning brush at 90 degree angle and insert the brush into the opening in the side of the suction valve housing. Use short strokes to advance the brush through insertion tube until it emerges from the distal tip. Remove any debris from the bristles with fingers, gently pull the brush back through the channel, inspect and clean bristles again. Repeat this process until debris can not be seen on the bristle tip. Inspect and clean bristles while submerged. l) Brush at 45 degree angle; insert the brush in the center of the suction valve opening. Pass the brush through the universal cord until it emerges from the suction connector. Clean the bristles and withdraw the brush. m) Brush the suction channel within the universal cord; insert the brush in the center of the suction valve opening. Pass the brush through the universal cord until it emerges from the suction connector. Clean the bristles and withdraw the brush. Continue brushing until there is no visible debris on the bristle tip. n) Clean the channel openings. Carefully insert cleaning brush into the suction valve housing. Rotate the brush and remove. Clean the bristles. Repeat this process until no visible debris remains. o) Clean the instrument channel port. Insert brush, rotate and remove and clean the bristles and repeat for a minimum of three times. Continue until no visible debris remains. Flush the channels with endoscope compatible detergent to remove any remaining debris. p) When all internal channels are filled with detergent, wipe external surfaces of the scope with a soft lint free cloth or sponge to remove any debris. q) Soak scope and accessories in detergent for the time recommended by the manufacturer. r) Discard endoscope compatible detergent after each scope. s) Prior to manual high level disinfection or with AER's that only high level disinfect, rinse all channels with tap water using irrigation adapters as needed following cleaning to remove all traces of detergent and debris. i. Attach suction tubing to suction port and aspirate approximately 500ml water through suction channel or use a syringe. ii. Dry all channels with regulated forced air and dry the exterior of the scope with a soft, lint-free cloth and all accessories before disinfecting to prevent dilution of the disinfectant. 9 NOTE: As an alternative to manual flushing, automated flushing may be accomplished via use of a preprocessing sink or automated flushing pump, e.g. Endo Flush Pump. This does not eliminate the requirement for manual brushing as detailed in 3h. If using an automated scope reprocessing machine, ensure that an endoscope compatible detergent approved by the scope reprocessing machine manufacturer is used for the cleaning cycle. 4. High Level Disinfection – Automated (AER) a) Perform hand hygiene per protocol. b) Wear PPE during all scope reprocessing steps; include nitrile gloves, impervious gown, hair covering, mask and eye protection such as safety chemical goggles. c) Ensure exhaust ventilation is operating before using disinfectants. d) Ensure disinfectant reservoirs are labeled with solution name, active ingredient, and primary hazards (e.g., “Caution: Cidex contains glutaraldehyde, which may cause respiratory system sensitization and contact dermatitis”), as well as the expiration date and the initials of the person who prepared the solution. e) At the beginning of the day visually confirm flow through all AER channel adapters prior to scope disinfection by running a diagnostic cycle (i.e. wash only cycle for a Custom Ultrasonic unit). f) Ensure disinfectant solution has met the minimum effective concentration (MEC) level. g) Use test strips following manufacturer instructions. Ensure that test strip container is tightly covered prior to and after use and that the test strips have not expired. Test strip containers should be labeled with expiration date 90 days from date of opening or what ever the manufacturer requires. Test high level disinfectant solution for minimum effective concentration (MEC) before each use, and document test results. If solution does not show adequate concentration, neutralize and discard. New solution must be used. The high-level disinfectant may never be used passed the expiration date. Any scope processed with expired solution must be recalled and reprocessed. h) Follow the AER manufacturer’s instructions for connecting the scope to the reprocessor. i) Place the valves and other removable parts in the reprocessor. j) Confirm that all documentation has been logged and/or has been entered. k) Start machine following AER manufacturer written instructions. Confirm cycle time is consistent with disinfectant label claims. l) At the completion of cycle, flush with 30ml alcohol for one scope and 60ml for two scopes. Purge with air through channels for a minimum of one (1) minute. m) Upon completion of cycle, verify that correct cycle parameters have been met. Initial documentation. High Level Disinfection or Sterilization – Manual a) Perform hand hygiene per protocol. b) Wear PPE during all scope reprocessing steps; include nitrile gloves, impervious gown, hair covering, mask and eye protection such as safety chemical goggles during scope reprocessing c) Ensure GUS (glutaraldehyde user station) or slot exhaust ventilation is operating before using disinfectants to reduce the exposure of employees to liquid chemical disinfectants. d) Ensure disinfectant secondary containers are labeled with solution name, active ingredient, and primary hazards (e.g., “Caution: Cidex contains glutaraldehyde, which may cause respiratory system sensitization e) and contact dermatitis”), as well as the expiration date and the initials of the person who prepared the solution. f) Ensure disinfectant solution has met the minimum effective concentration (MEC) level. g) Use test strips following manufacturer instructions. Ensure that test strip container is tightly covered prior to and after use and that the test strips have not expired. Test strip containers should be labeled with expiration date 90 days from date of opening or what ever the manufacturer requires. Test high level disinfectant solution for minimum effective concentration (MEC) before each use, and document test results. If solution does not show adequate concentration, neutralize and discard. New solution must be used. The high-level disinfectant may never be used passed the expiration date. Any scope processed with expired solution must be recalled and reprocessed. h) Use scope manufacturer specific cleaning tubing adaptors or syringes to fill all channels completely until no bubbles are seen. All channels must be reprocessed, even if not used in a given procedure. i) Place all valves and reusable parts in disinfectant solution. j) Two percent (2%) glutaraldehyde solutions require a minimum of 20 minutes soak time at 20 C (68 room temperature). Submerge and soak the scope and accessories in high level disinfectant. Soak time begins after full immersion and all channels are completely filled with high level disinfectant. k) Refer to manufacturer’s guidelines/recommendations for disinfectants other than Cidex. l) Wipe the soaking container exterior with an approved hospital disinfectant wipe after placing scope to soak. 10 Rinsing/Drying a) Perform hand hygiene per protocol. b) Wear clean PPE once the scope has been high-level disinfected; include nitrile gloves, impervious gown, hair covering, mask and eye protection such as safety chemical goggles. c) Manual Rinsing: pour copious amounts of water over exterior of scope three times and flush all lumens three times with 100ml each rinse/each lumen or rinse under running water for one minute and flush all lumens three times with 100ml each rinse. d) After final tap water rinse, flush all channels, including accessory channels with 5ml, with 70% ethyl or isopropyl alcohol until the alcohol can be seen exiting the opposite end of the channel to facilitate drying. Visually check that each ports has flow during alcohol purge. Check with scope manufacturer for model specific information. e) Purge all channels with regulated forced air including insertion tube and inner channels. f) Remove all cleaning adapters and devices. g) A new syringe should be used each day for alcohol flushing. Date and initial syringe. The tubing adapters should be high level disinfected once a day. h) Dry the exterior and accessories with a soft, dry lint-free cloth. i) Remove personal protective gear. j) Wash hands before leaving reprocessing room. MANUAL DISINFECTION Immerse the endoscope in compatible detergent Use the suction cleaning adapters to fill all channels completely until no bubbles are seen. Place all valves and reusable parts in endoscope compatible detergent. All high-level disinfection containers must be labeled with solution name, active ingredient, primary hazard, expiration date and initials of person who prepared the solution. Keep containers tightly covered. Follow manufacturer’s high level disinfectant instructions for the immersion time and set timer. All channels must be reprocessed even if not used in a given procedure. AUTOMATICE REPROCESSOR (AER) Attach AER manufacturer channel cleaning adapters. Follow the AER manufacturer’s instructions to connect the scope to the reprocessor. Place the valves and other reusable parts in the reprocessor. At the beginning of the day, visually confirm flow through all channel cleaning adapters by running a diagnostic “wash only” cycle. For Custom Ultrasonic AER’s, confirm the “wash/disinfect” cycle is selected for all scopes that will not be terminally sterilized. Attach the GI auxiliary water channel or the Duodenoscope/ERCP elevator wire channel cleaning adapter to the high pressure ports in the CU AER. Refer to manufacturer’s instructions for high level disinfectant use life and contact time. Label disinfectant reservoir with solution name, active ingredient, primary hazard, expiration date and initials of person who prepared the solution. Verify the machine setting for the recommended time and correct cycle. Only the service tech may change cycle settings. Validation of completed cycle for Custom Ultrasonic AER’s as follows: o Enter patient name, MRN, scope type and serial #, physician, procedure, and technician information, o Print cycle validation ; check and place signature on printout of each cycle completed correctly o Save and/or clear cycle and re-set for next cycle Look at scopes to visually check flow of fluids through channels during the final alcohol rinse step. All channels must be reprocessed even if not used during a given procedure. 7. Storage Channeled scopes after high level disinfection: Hang scopes vertically in a ventilated cabinet specifically designed for scopes, to facilitate drying. Remove control valves, distal hoods, caps, etc., and store removable parts separately. If storing fiberoptic scopes the EtO valve should be in place. To protect scopes from incurring damage during storage, the number of scopes should not exceed the number of scope holders provided by the cabinet manufacturer. Cabinet fan must be in the ON position. Fan is only activated when doors are closed. Doors must remain closed at all times. NOTE: If scope is not stored per these guidelines, scopes must be reprocessed just prior to use. a) Channeled scopes that have been sterilized (e.g. EtO, Sterrad NX) will be stored in sterile wrapping/package. b) Non-channeled scopes: After thoroughly drying, may be stored in a manner that prevents recontamination before next use. Vertical storage not required. c) Clean or sterile gloves must be worn at all times when storing, removing or handling high-level disinfected scopes. d) Scopes should be reprocessed if stored for > 30 days or if integrity of scope is compromised. 11 e) Any scope removed from a cabinet and not used must be returned to the reprocessing room. f) A closed container should be used if transporting HLD scopes between rooms, e.g. from reprocessing area to storage cabinets that may be in the procedure rooms. g) Decontaminate soiled scope transport containers after each use using a manufacturer approved disinfecting product. NOTE: A system should be in place to clearly identify which scopes have been completely reprocessed. This is recommended in order to prevent a scope being used in more than one procedure without reprocessing between uses. 8. Biopsy Forceps a) Soak reusable biopsy forceps in a compatible detergent as soon as possible after procedure. b) After each patient use, assure that biopsy forceps are cleaned and sterilized following the device manufacturer validated instructions for use (IFU). c) Disposable biopsy forceps are not to be reprocessed. Dispose of single-use biopsy forceps into a sharps container. 9. Water Bottle a) Sterilize or high-level disinfect both the water bottle used to provide intra-procedural flush solution and its connecting tube after each patient, if using reusable. After sterilizing or high-level disinfecting the water bottle, fill with sterile water. NOTE: The use of a disposable system is recommended. Review all manufacturer instructions for reprocessing reusable accessories used in the procedure room. 10. Other Accessories a) Sterilize or high-level disinfect all non-disposable semi-critical items (items that touch intact mucous membranes) with an approved high-level disinfectant. Follow high-level disinfection with three water rinses. i. Suction Adapter, Air- Water Adapter, Biopsy Adapter, and Door Clamp – large or small types. ii. Sterilize all non-disposable critical items (items that enter sterile tissue). NOTE: When using other high level disinfectants, follow manufacturer’s directions. 11. Processing Area a) Cleaning/disinfection areas should be separate from patient care and supply/storage areas. b) When using the same sink for manual reprocessing, wipe counter and sink with a hospital approved surface disinfectant between cleaning and final rinse cycles to avoid recontamination of scope. c) Disinfect the lid and keyboard of the AER to avoid recontamination of HLD scopes prior to removal. d) Clean and disinfect any reusable wash and rinse basin used for manual reprocessing at the end of the day. 12. Personal Protective Equipment (PPE) a) Gowns referenced in this document are impervious gown, nitrile, chemotherapy or butyl gloves referenced in this document are nitrile or butyl rubber gloves, which cover above the wrist. Other PPE referenced in this document include mask, eye protection such as chemical splash goggles or a full face shield or combination shield/mask, and hair covering. b) Personnel with open skin lesions should not perform or assist with any scope procedure or handle equipment used for procedures. c) Wash hands after each patient procedure, after each glove removal, and when entering and leaving endoscopy suite or endoscopy reprocessing area. 13. Record Keeping and Preventative Maintenance a) Record high level disinfectant concentration monitoring results before each use in logbook. b) A log must be maintained indicating for each procedure the patient’s name, medical record number, date, procedure type, scope type, and scope serial number or other identifier, endoscopist, the AER and cycle used (see sample log). c) Record all filter changes to assure they are changed regularly per manufacturer recommendations. (See Preventative Maintenance Checklists). d) Notify local Infection Control Practitioner and Risk Management if reprocessing deficiencies are identified so that they can determine if DPH should be contacted. 12 Quick Reference Grid Scopes With Channels Female Reproductive Hysteroscope Hysteroscope used with Essure implant Gastrointestinal Colonoscope Duodenoscope ERCP (Endoscopic Retrograde CholangioPancreatography) Gastroscope Sigmoidoscope EUS scope Genitourinary Cystoscope Ureteroscope Flexible Rigid NOTE: Flexible Cystoscope cannot be HLD with Cidex OPA Pulmonary Bronchoscope Intubation Laryngoscope (i.e. Olympus LF-GP) Point of Use Pre-cleaning, Leak Testing and Cleaning 1. Immediately wipe outside of scope and flush channels with compatible detergent. 2. Leak test - visually inspect for holes, tears or damage. Detach valves. NOTE: For cystoscopes follow manufacturer recommendations regarding whether or not to do leak testing. 3. Immerse the scope into the endoscope compatible detergent. Wash all debris from the exterior by brushing and wiping the instrument while submersed in solution. 4. Detach suction, biopsy channel cover, and all other removable parts; use a brush to clean valves and biopsy port openings. 5. Brush the entire suction/biopsy system (should be submerged). After each passage rinse the brush before retracting it and before reinserting it. Continue brushing until no debris is left on the brush Examine brush after use to ensure it is intact. 6. Rinse all channels thoroughly with compatible detergent, followed by tap water and then forced air-drying. Towel dry exterior. 7. After manual brushing as an alternative to manual flushing, automated flushing may be accomplished via use of a “pre-processing sink” or endo flush pump. 8. If using reusable biopsy forceps separately soak biopsy forceps in compatible detergent; then use ultrasonic cleaner prior to sterilization, rinse, dry and package for sterilization. 9. Abdominal Cavity Laparoscope Orthopedic Arthroscope Urology Nephroscope Scopes w/o Channels Scopes entering sterile tissue should be sterilized (e.g. hysteroscope, cystoscope). If this is not possible then they should be high level disinfected. The methods for both are as follows: Sterilization: EtO (Ethylene Oxide) Sterrad 100S (Hydrogen Peroxide Plasma) – for rigid scopes only. Sterrad NX & 100NX (Hydrogen Peroxide Plasma) – for scopes with one channel only (HNS, OB/GYN, UROLOGY) High Level Disinfection (HLD) - Manual or Automated: Manual: Soak in high level disinfectant1 solution for designated time frame (e.g., 20 minutes for 2% glutaraldehyde); rinse all channels with sterile water, or tap water followed by (Manual/Automated): three rinses for manual disinfection – exterior of scope plus 100mL water each lumen/each rinse; two rinses for automated disinfection – default cycles; dry channels with 5mL of 70% alcohol rinse per lumen and forced air. For automated disinfection visually confirm flow through all ports prior to reprocessing scopes. For automated disinfection machines with both buttons, ensure that the “disinfect/wash” and not the “wash” cycle is selected. NOTES: Flexible Cystoscope cannot be HLD with Cidex OPA. Biopsy forceps must be steam sterilized. As above Sterilization only As above Sterilization Only As above Point of Use Pre-cleaning, Leak Testing, Cleaning Sterilization Only 1. Upper Airway/Digestive (HNS, Anesthesia) Nasolaryngoscope Nasopharyngeal scope During cleaning flush elevator wire channel of duodenoscopes/ERCP scopes and GI scopes with an auxiliary water channel Rinsing, Disinfection, and Sterilization Immediately wipe outside of scope with compatible detergent Leak test per manufacture recommendationvisually inspect for holes, tears or damage. Immerse the scope into the compatible detergent solution. Wash all debris from the exterior by brushing and wiping the instrument while submersed in solution. Rinse thoroughly with tap water and towel dry. Disinfection, Rinsing, Sterilization Scopes should be sterilized. If this is not possible then HLD at a minimum. Sterilization: EtO (Ethylene Oxide) Sterrad 100S (Hydrogen Peroxide Plasma) – for rigid scopes only. Sterrad NX (Hydrogen Peroxide Plasma) – Advance Cycle only – flexible scopes only High Level Disinfection: Soak in high-level disinfectant1 solution for designated time frame (e.g., 20 minutes for 2% glutaraldehyde); rinse with tap water (three rinses for manual disinfection; automated rinses are preset. Wipe scope with a lint free alcohol cloth as final step. Store dry between uses. 13 7. APIC Guidelines APIC Guidelines can be downloaded from the following website: http://www.apic.org/AM/Template.cfm?Section=Guidelines&CONTENTID=6381&TEMPLATE=/CM/ContentDisplay.cfm or can be referenced in the journal where originally published: AJIC Am J Infect Control 2000; 28:138-55. 14 See appendix 8. SGNA Chart Print and Add a copy of the latest SGNA Guidelines here. As of this printing, the latest version is 2012. “Steps Necessary to Thoroughly Clean and High Level Disinfect Flexible Endoscopes”, can be purchased from SGNA for $50.00 each. Orders can be placed on-line using their web site: http://www.sgna.org or by contacting them directly at: Society for Gastroenterology Nurses and Associates, Inc. 401 North Michigan Avenue Chicago, IL 60611-4267 Voice: 800-245-7462; in Illinois: 312-321-5165 FAX: 312-527-6658 15 KAISER PERMANENTE Program-wide Scope Reprocessing Handwashing Protocol Employee’s name (PRINT): Date: Department: □ Initial Competency Facility: □ Annual Competency Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level Hand washing: 1. Hands are washed prior to donning PPE. NOTE: Alcohol-based degermers may be used when hands are not visibly soiled. 2. Hands are washed when gloves fail, e.g., hole/torn glove. 3. Hands are washed prior to leaving the reprocessing area. 4. Hands are washed upon returning from break, restroom, or meal break. 5. Hands are washed after removing PPE. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: _____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: ______________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:______________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:_____________ Educational Plan: ________________________________________________________________________ Employee signature for acknowledgment of plan: _______________________________________________ 16 KAISER PERMANENTE Program-wide Scope Reprocessing All flexible scopes Point-of-Use precleaning Procedure Room/Department Employee’s name (PRINT): Department: Date: Facility: □Initial Competency □ Annual Competency Skill Level: 1 -Novice/in training 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level Point of Use Pre-Cleaning: Patient Procedure Room/Department NOTE: pre-cleaning is a critical step in ensuring appropriate high-level disinfection. Any body fluids or debris left on the instrument may prevent adequate disinfection. Perform hand hygiene per protocol. 1. Wear PPE including (at a minimum) gown, gloves, hair covering, and eye protection such as faceshield or chemical splash goggles. 2. Follow manufacturer dilution instructions for and endoscope compatible detergent solution. Another option is to use a packaged premixed kit, (e.g., FIRST STEP) or, if mixing and diluting a solution care should be taken to avoid splashing or aerosolization of the product. 3. Immediately following procedure, with the scope still connected to the light source, wipe the exterior of scope with freshly prepared compatible detergent or enzymatic solution lint free cloth. 4. Place the distal end of the insertion tube into the compatible detergent or enzymatic solution and with the biopsy port inlet covered, suction detergent up through the instrument channel for several seconds. Suction/flush with compatible detergent or enzymatic solution through the scope until the solution is visibly clear. Alternate between suctioning detergent and air to create agitation and to enhance cleaning. Finish by suctioning the instrument channel with air. 5. Depress the air/water adapter button during flushing of channels. 6. Clear the air and water channels according to manufacturer’s instructions. 7. For Duodenoscopes/ERCP scopes, flush elevator wire channel with compatible detergent solution, as radiographic media and bile can dry quickly. 8. Disconnect the scope from the light source (and suction source). 9. Auxiliary Water Tube (Olympus) is to be sent with scope for cleaning and sterilization after each use, per manufacturer instructions. 10. Endo Smart Cap and EndoGator disposable tubing systems are to be discarded every 24 hours per manufacturer instructions. 11. Water Bottles are to be discarded every 24 hours per manufacturer instructions. 12. Transport scope and all reusable accessories, in covered appropriately labeled container to reprocessing area. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: _____________________________________________________ Date: _______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _______________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:_______________ Print: ______________________________________________ 17 FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ ____________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:_____________ Educational Plan: _____________________________________________________________________ Employee signature for acknowledgment of plan: __________________________________________________________________ 18 KAISER PERMANENTE Program-wide Scope Reprocessing Dry and Wet Leak Testing Employee’s name (PRINT): Date: Department: □Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level Dry and Wet Leak testing (if applicable) in reprocessing room: (after each patient use) 1. Wear PPE including impervious gown, nitrile, chemotherapy or butyl gloves, mask, faceshield or, chemical splash goggles and hair cover throughout the cleaning process. 2. Assure water resistant cap is connected. NEVER connect or disconnect water resistant cap while the scope or cap is immersed as it will damage the scope. Assure venting cap is detached. 3. Prior to immersing scope in any fluid, visually inspect the scope for holes, tears, or other gross damage. 4. Detach all removable parts. 5. Turn on leakage tester if powered. Depress pin inside leak tester to ensure air flow. Attach leakage tester and pressurizer to scope. 6. Inspect the bending rubber to insure the scope is pressurized – either visually, or by touch. While angulating the tip in all directions, observe leak tester gauge. Pressure should remain in the green zone on manual leak tester - Approximately 30-60 seconds 7. Submerge the entire scope for a minimum of 30-60 seconds in clean water. Curl the light guide and insertion tube and look for a continuous stream of bubbles. Again while angulating the distal tip in all directions look for leaks. Check the following areas: Control knob, Insertion tube, including the distal tip and the connectors. 8. If no leak is detected remove scope from water, release air pressure and disconnect leak tester. 9. When a leak is detected, follow manufacturer’s recommended procedure for disinfecting the scope. Remove scope from service and send for repair. If scope is not properly disinfected, it should be considered a contaminated medical device by attaching biohazard and supplemental label “contaminated medical device”. 10. Clin Tech, Periop, or Supply Chain Management arrange for flexible and rigid scope repairs with designated repair vendor. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: _____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: ______________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:_______________ Print: ______________________________________________ 19 FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _______________________________________________ Manager Follow-up:______________________________________________________Date:_______________________ Educational Plan: _____________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 20 KAISER PERMANENTE Program-wide Scope Reprocessing Manual Cleaning Employee’s name (PRINT): Department: Date: Facility: □Initial Competency □ Annual Competency Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level Manual Cleaning: 1. Fill the sink with freshly prepared endoscope compatible detergent solution properly diluted according to manufacturer instructions. Completely immerse the scope. 2. Use a sponge or lint- free cloth to thoroughly clean the exterior of the scope. 3. Use a small soft brush to clean removable parts and inside the suction valve, air water valve biopsy port openings and all other channel openings. Keep the scope submerged during cleaning to prevent splashing and aerosolization of contaminated fluid. 4. For the Duodenoscope/ERCP scope, deflect the elevator wire channel and brush thoroughly. 5. If the scope features an instrument channel, detach the biopsy port cap (or seal) and brush and wash the channel by advancing the appropriately sized brush down through the channel via the biopsy port inlet. When it is seen exiting the scope’s distal tip, rinse the brush to remove any visible debris. Retract the brush up through the channel, remove it, rinse it, and reinsert it. Repeat this step three times or until there is no more visible debris on the brush. 7. For manual flushing, attach scope manufacturer cleaning adapters to the scope and cover the biopsy port. Flush all channels with detergent solution as recommended by the scope manufacturer and soak for the time recommended by the detergent manufacturer. 8. For manual flushing of ERCP scopes with an elevator wire channel or GI colonoscopes or gastroscopes with an auxiliary water channel, attach a syringe and injection tube to flush detergent through these special channels. 9. For automated flushing using the Custom Ultrasonic preprocessing sink, attach the CU specific tubing adaptors and clamp to the scope. Use the high-pressure ports to flush the elevator wire channel and the auxiliary water channel. Flush all channels with detergent solution and soak for the time recommended by the detergent manufacturer. All channels must be processed whether they were used or not. 10. Discard detergent solution and disposable brushes after each use. If reusable, they must be thoroughly cleaned and high level disinfected or sterilized after each use (discard brushes if unable to clean or when they show signs of wear). 11. Rinse the exterior of the scope and all removable parts thoroughly with tap water to remove residual detergent solution. 12. Flush clean water through all channels to remove any residual detergent solution. 13. Flush air through all channels. 14. Use a lint-free cloth to remove excess moisture prior to placing in disinfectant. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: _____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: ______________ Print: ______________________________________________ 21 Manager Signature: ______________________________________________________Date:______________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow up: ______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ ___ Employee signature for acknowledgment of plan: ______________________________________________________ 22 KAISER PERMANENTE Program-wide Scope Reprocessing HLD MEC Testing, Activation and Deactivation Employee’s name (PRINT): Department: Date: Facility: □Initial Competency □ Annual Competency Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level High-level Disinfectant – Testing the Minimum Effective Concentration 1. Wear PPE including impervious gown, nitrile, chemotherapy or butyl gloves, mask, hair cover and mask/faceshield. NOTE: Nitrile, chemotherapy or butyl gloves are required when working with HLD. 2. Test the high-level disinfectant solution before each use to be sure it is above the minimum effective concentration level. Log results NOTE: If disinfectant fails early, notify Department Manager. 3. QA testing is performed on every newly opened bottle of test strips and every 30 days for any unused test strips using a new bottle of HLD. 4. Test strip container must be dated with the open and expiration date. The expiration date does not exceed the shelf life date. 5. HLD is not used passed its use life, e.g., Activated Cidex is good for 14 days, even when the test shows the HLD meets the MEC level. High-level Disinfectant - Activation and Deactivation 1. Wear PPE including impervious gown, nitrile, chemotherapy or butyl gloves, mask, hair cover and chemical splash goggles. 2. Reprocessing rooms equipped with Nederman arm exhaust systems have the arms pulled down below the breathing zone when pouring HLD into the reservoir. 3. The date and the lot number of the HLD are logged when the HLD is changed. 4. The MEC level is tested prior to using the new HLD and results logged. 5. A bottle of HLD that has not been emptied has been appropriately labeled with the expiration date. 6. Empty bottles of HLD are placed in regular trash receptacles. 7. Dispose of expired disinfectant solution AFTER neutralizing as directed by local Safety Dept: Pour in the appropriate amount of glyciene neutralizer 8. Allow to sit for the required time and confirm color change for neutralization completion. 9. Demonstrate that neutralization occurs by testing MEC and placing results in the logbook: Date neutralized, amount neutralized and initial of the person completing this task. 10. Thoroughly rinse AER and run a “wash” only cycle to eliminate any residual neutralizing product. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: _____________________________________________________Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ 23 Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 24 KAISER PERMANENTE Program-wide Scope Reprocessing High-level Disinfection - Manual Employee’s name (PRINT): Date: Department: □ Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level Manual Scope Disinfection 1. 2. 3. 4. 5. 6. Remove venting cap prior to immersion in the disinfectant. Attach injection tube to cleaned scope and immerse in disinfectant. Use a syringe to completely fill channel/s with disinfectant solution. Place removable parts in disinfectant solution. While the scope is immersed, disconnect injection tube. Cover the disinfectant solution tray securely and soak device for the time specified by manufacturer’s instructions or KP policy (e.g. for 2% glutaraldehyde a minimum of 20 minutes). 7. Don clean PPE. 8. Connect injection tube with syringe and purge air to remove disinfectant from channel/s. 9. Thoroughly rinse the exterior surface with copious amounts of sterile water or tap water (three water rinses of exterior of scope or amount recommended by manufacture per size of scope). 3-2-1 rinse means three rinses with two gallons of water for one minute soak time. NOTE: Contact with enzymatic detergents’ and high level disinfectants’ liquid and vapor can severely irritate the eyes and mucus membranes, and at higher concentrations burns the skin. Discard water after each scope. 10. Purge water from channel/s using a syringe or medical grade air. 11. Flush with 70% isopropyl alcohol, 5 ml per channel. 12. Dry the exterior with a soft, dry lint-free cloth. 13. Remove PPE. 14. Scopes that are used for sterile procedures must be reprocessed prior to use: Must include pre-cleaning through the disinfection and rinse steps. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: ____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 25 Manager Follow-up:______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 26 KAISER PERMANENTE Program-wide Scope Reprocessing Staff Competency Evaluation Tool CUSTOM ULTRASONICS – DOS Operating System Employee’s name (PRINT): Department: Date: Facility: □ Initial Competency □ Annual Competency Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Competency Performance Check List “MET” means the individual independently performs each responsibility AER Reprocessing – DOS Operating System Method Skill Level 1. Check the Custom Ultrasonic approved detergent level. Replace the detergent when the level is no less than one inch from the bottom of the bottle. 2. As the first cycle of the day, attach all Custom Ultrasonic tubing adapters to the AER and run a diagnostic cycle to ensure flow through all channels. Use the F6 “wash only” selection. 3. Follow the Custom Ultrasonic instructions to connect the scope to the reprocessor. 4. Ensure the waterproof cap and the valve cover (door clamp) are attached to the videoscope. Ensure suction button is held open using the suction button cleaning adapter. 5. Place the scope in the processing chamber & attach the appropriate tubing adapters as follows: Air adapter to the air port, water adapter to water port, suction adapter to suction port, biopsy adapter to biopsy port, high pressure tubing to the auxiliary or elevator port. 6. Ensure all adapters are laying flat & nothing is sticking out of machine. 7. Wipe top surface area of bay with a hospital approved disinfectant and close lid. 8. Hold down the ALT button and press the letter U. 9. Single chambered system: scroll through guidelines and presses ENTER. 10. Divided screen: highlight the desired bay # using left and right arrow keys, press ENTER after selecting. 11. a) Type patient’s name, press TAB key. b) Type Patient’s MRN, press TAB key. c) Use arrow key until provider’s name is highlighted, press ENTER. d) Use arrow key until scope # is highlighted, press ENTER. e) Press TAB & ENTER, repeat above steps for 2nd patient. f) Upon completion hold down the ALT key & press O key. NOTE: this will exit the “Patient List and System Data” screens. 12. Press F8 “Wash & D/S/P (High level disinfectant). 13. Verify the D/S/P Level is above the Low Level mark and press F1. 14. Confirm the flow of HLD through the channels. 15. Prior to removing soiled PPE, wipe AER lid exterior and keyboard with a hospital approved disinfectant wipe. 16. At completion of cycle and prior to disconnecting scope: a) Don clean PPE b) Confirm tubing adapters remained connected and not kinked during processing. c) Verify printout for correct cycle parameters. If Error is noted: scope must be reprocessed. d) With lid closed, purge scope with 70% Isopropyl alcohol: 30ml for one scope or 60ml for two. e) Turn “On” the air switch (checks for light indicating pump operating). Run for a minimum of one minute. f) Turn the Air switch “Off”. 27 17. At completion of program follow the screen prompt to F2 to print receipt and F3 to finish. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: ____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 28 KAISER PERMANENTE Program-wide Scope Reprocessing Staff Competency Evaluation Tool CUSTOM ULTRASONICS – Windows Operating System Employee’s name (PRINT): Department: Date: Facility: □ Initial Competency □ Annual Competency Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Competency Performance Check List “MET” means the individual independently performs each responsibility AER Reprocessing –Windows Operating System Method Skill Level 1. Check the Custom Ultrasonic approved detergent level. Replace the detergent when the level is no less than one inch from the bottom of the bottle. 2. As the first cycle of the day, attach all Custom Ultrasonic tubing adapters to the AER and run a diagnostic cycle to ensure flow through all channels. Use the F7 “wash only” selection. For AER’s with channel monitoring systems, ensure all adapters are connected. 3. Follow the Custom Ultrasonic instructions to connect the scope to the reprocessor. 4. Ensure the waterproof cap and the valve cover (door clamp) are attached to the videoscope. Ensure suction button is held open using the suction button cleaning adapter. 5. Place the scope in the processing chamber & attach the appropriate tubing adapters as follows: Air adapter to the air port, water adapter to water port, suction adapter to suction port, biopsy adapter to biopsy port, high pressure tubing to the auxiliary or elevator port. 6. Ensure all adapters are laying flat & nothing is sticking out of machine. 7. Wipe top surface area of bay with a hospital approved disinfectant and close lid. 8. TAB to CU system and press ENTER. 9. Single chambered system: scroll through guidelines and press ENTER. 10. Divided screen: highlight the desired bay # using left and right arrow keys, press ENTER after selecting. 11. a) Type patient’s name, press TAB key. b) Type Patient’s MRN, press TAB key. c) Use arrow key until provider’s name is highlighted, press ENTER. d) Use arrow key until scope # is highlighted, press ENTER. e) Press TAB & ENTER, repeat above steps for 2nd patient. f) Upon completion hold down the ALT key & press O key. NOTE: this will exit the “Patient List and System Data” screens. 12. Press F6 “Wash & Disinfect” cycle. 13. Verify the Disinfectant Level is above the Low Level mark and press F1. 14. Confirm the flow of HLD through the channels. 15. Prior to removing soiled PPE, wipe AER lid exterior and keyboard with a hospital approved disinfectant wipe. 16. At completion of cycle and prior to disconnecting scope: a) Don clean PPE b) Confirm tubing adapters remained connected and not kinked during processing. c) Verify printout for correct cycle parameters. If Error is noted: scope must be reprocessed. d) With lid closed, purge scope with 70% Isopropyl alcohol: 30ml for one scope or 60ml for two. 29 e) Turn “On” the air switch (checks for light indicating pump operating). Run for a minimum of one minute. f) Turn the Air switch “Off”. 17. At completion of program follow the screen prompt to F3 to print receipt and F5 to finish. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: ____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 30 KAISER PERMANENTE Program-wide Scope Reprocessing Staff Competency Evaluation Tool Medivators – Advantage Plus Employee’s name (PRINT): Date: Department: □ Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Competency Performance Check List “MET” means the individual independently performs each responsibility Daily checks prior to running first cycle of the day Method Skill Level 1. Verify machine is “On” 2. Open water shutoff valve to the incoming water line. 3. Check the expiration date of the HLD (Rapicide PA) – use life is 21 days once opened. 4. 5. 6. 7. Check Rapicide PA, parts A and B, container levels. Check detergent level. Check alcohol level. Confirm date and time on display are correct. 8. Check drain filters and replace if excessively soiled. 9. Check “O” rings on the connection manifolds to verify they are not missing and they are not damaged. 10. Inspect and confirm that all Advantage Plus connector blocks are in good working order (i.e., no holes, kinks). Do not modify any connector blocks. 11. Confirm pressure controls are at acceptable pressure ranges: Main air: 42 45 psi Leak test air pressure: 3.6 – 3.75 psi Channel connectivity: 25.5 - 30 psi Placing endoscope into the Advantage Plus 12. Place endoscope into the basin with the control knobs facing up, in left front corner. 13. Set the insertion tube in a clockwise circle in the basin. 14. Next fit the light guide tube in a counterclockwise circle in the basin. 15. Select the proper connection block and channel separator from the endoscope hookup guide and attach to scope. 16. Position the channel separator adapter so the O-ring fits into the water/air valve. 17. Hold the connector block with the handle positioned to the right and place the block lightly over the manifold, making certain there are no kinks in any of the hoses. 18. Ensure the connector block is firmly attached to the manifold of the basin by moving the handle to the left and locking it in place. 19. Place cleaned valves in accessory bag and tuck under endoscope. 20. Prior to removing PPE, wipe lid exterior and keyboard with a disinfectant wipe. 21. Next, press the Menu button and select log in name by using the Scroll Down button or scanning operator’s barcode and click OK. 22. When Main Menu window opens, highlight Endoscope Selection, click OK 23. From the Hookup Menu window, scan the barcode on the block and click OK From the Endoscope Section window, scan barcode on the endoscope and click OK. 31 24. Close lid using the foot pedal or by pressing the Open/Close 25. When prompted enter: button. Provider – Click OK Patient MRN – Click OK 26. Cycle will automatically start. 27. When cycle is complete, follow screen prompts and test MRC. 28. Follow the screen prompt, test the HLD and indicate if it passed or failed the MRC. 29. Pressing the Open/Close button. When prompted, use Scroll Down button or scan operator’s barcode and click OK. . Lid will open. 30. Confirm that all adapters are still connected to the channel connections and cycle parameters were met. 31. Don clean PPE before removing scope. 32. Disconnect scope from cleaning accessories and remove from basin. 33. Wipe with a clean lint-free cloth 34. Use soap and water to clean the lid gasket to prevent sticking Maintenance checks 35. Filter changes (inlet water supply is turned off prior to changing filters): 0.1 Micron – every 6 months 0.4 Micron – every 3 months 1.0 Micron – every 3 months 36. Air filters x4 are changed every 6 months 37. Water line and water filter disinfection is performed after the 0.1 filter is changed every 6 months 38. Pull and check the basin drain monthly for wear and debris Overall Evaluation of Demonstration Meets expectations Observer/Proctor: ____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: _____________________________________________________ 32 KAISER PERMANENTE Program-wide Scope Reprocessing Scope Storage Employee’s name (PRINT): Date: Department: □ Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level Storage: 1. All disinfected scopes must be handled with clean or sterile gloves to reduce recontamination of the scope. 2. Disinfected scopes should be used immediately, or stored in a manner to minimize recontamination. High-level disinfected label must be attached to each scope identifying expiration date. Reprocess if exceeds 30 day expiration date. 3. Decontaminate transport container between scopes using a manufacturer approved product. 4. Hang all channeled flexible scopes vertically in a ventilated closet or cabinet. Never store in carrying case. NOTE: If not stored appropriately, scope must be reprocessed prior to use beginning with manual cleaning. 5. Store removable parts separately (not attached to scope), i.e., buttons and valves. 6. HLD Scopes used for sterile procedures must be reprocessed prior to use: a) Must reprocess beginning with manual cleaning step. 7. Scope cabinets are on a routine cleaning schedule and cleaning is documented. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: ____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:____________________ 33 Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 34 Kaiser Permanente Program-wide Scope Reprocessing Record Keeping and Documentation Employee’s name (PRINT): Date: Department: □ Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Performance Criteria 1. Method Skill Level Ensure that all documentation is complete: (All elements below must be documented). a) Date, time and type of procedure b) Patient’s name and medical record number c) Physician name d) Specific procedures(s) e) Scope serial number or other identifier. f) Type of scope g) Time In / Time Out of disinfectant (glutaraldehyde) if manually processing h) Operator initials 2. HLD validation sticker is attached to each processed item. 3. Scopes are reprocessed when there is no sticker present or when the scope is compromised. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: ____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 35 KAISER PERMANENTE Program-wide Scope Reprocessing Single Channel Endoscopes Sterilization with Sterrad NX/100 NX Employee’s name (PRINT): Date: Department: □ Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level Packaging: 1. Items which can be disassembled should be disassembled for sterilization. 2. 3. 4. 5. Attach venting cap to scope. A chemical indicator strip must be placed inside the package. Scope must have an external chemical indicator such as Sterrad indicator tape. Wrapped items should not have any gaps or openings. Peel packs are not permitted inside any wrapped tray. 6. Load Control Sticker should be applied after Sterrad sterilization cycle is complete. Biological Monitoring: 1. Perform Biological Monitoring each day the sterilizer is used. 2. Obtain a STERRAD CycleSure Biological Indicator (BI), peel pack in a Tyvek/Mylar pouch. Place BI test pack in the back/rear on the bottom shelf toward the back chamber wall. 3. Sterile only one (1) single-channel flexible scope at a time no other device may be run. 4. Start sterilization cycle. Run on the Advanced Cycle ONLY DUO or Flex Cycle for 100NX, where available Approved single-channel flexible scope lumen dimensions are: Single-channel flexible scopes with a polyethylene or Teflon® lumen, inside diameter of 1 mm or larger and length of 850mm or shorter. Please refer to the Sterrad Sterility Guide for a list of NX compatible flexible scopes. http://www.sterradsteriityguide.com ASP Customer Care Center : 1-888-STERRAD (1-888-783-7723) 5. After the sterilization cycle is complete, immediately remove the BI Test Pack from the sterilizer. Wear goggles and gloves when handling BI. 6. Check the chemical indicator for color change from red to yellow (STERRAD). Press cap down until firmly seated in order to seal vial while BI is inside the peel pack. 7. Remove BI from peel packaging. Using the tube crusher, squeeze the vial until glass media ampule has been crushed. Do not crush by hand because the broken glass may penetrate the BI vial and lacerate fingers. 8. Keep vial in a vertical position after it has been crushed. 9. Label vial with date and load number and places in incubator. 10. Incubate BI in an approved incubator at 55-60 degrees Centigrade for (24) hours (STERRAD). At this time the temperature of the STERRAD incubator will be recorded. 11. Crush an unprocessed vial to serve as a test Control, label with date, and place in same incubator as the processed vial. 11. After twenty four (24) hours (STERRAD) interprets results and record in log book. Discard the ampules in a biohazardous sharps container. Manufacturer’s Manual: 1. Sterrad NX Manual is available in department and staff can locate. 36 Policy/Performance Criteria Method Skill Level Maintenance: 1. ASP service technicians are responsible for preventive maintenance of Sterrad sterilizers. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: ____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 37 KAISER PERMANENTE Program-wide Scope Reprocessing Chemical Spill Response Employee’s name (PRINT): Date: Department: □ Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration Policy/Performance Criteria Method Skill Level Spill Procedure: Rationale: Ensure protection of staff and member from exposure to hazardous agents. 1. Follows Medical Center specific spill policy and procedure. 2. Following the process: S = Safety: wear PPE including chemical splash goggles, nitrile, chemotherapy or butyl gloves and non permeable gown and use the chemical spill kit to neutralize and contain the spill. I = Isolate: leave the department and close the door to prevent others from entering the area. N = Notify: hospital operator will notify the appropriate person(s) e.g. chemical spill team which should include Environmental Health & Safety. Overall Evaluation of Demonstration Meets expectations Observer/Proctor: ____________________________________________________ Date: ______________ Print: ______________________________________________ Employee Competency Signature: ________________________________________ Date: _____________ Print: ______________________________________________ Manager Signature: ______________________________________________________Date:____________ Print: ______________________________________________ FOR PROCESS IMPROVEMENT PURPOSES – ACTION PLAN List criteria needing review _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ Manager Follow-up:______________________________________________________Date:____________________ Educational Plan: _______________________________________________________________________________ Employee signature for acknowledgment of plan: ______________________________________________________ 38 KAISER PERMANENTE Glutaraldehyde Disinfectant Skills Validation Employee’s name (PRINT): Date: Department: □ Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration CRITICAL ELEMENTS Method Skill Level COMMENTS 1. Don personal protective equipment (gloves, hair cover, chemical splash goggles & fluid repellent gown). 2. Pre-soak, manually clean, rinse and dry instruments per Scope Competency skills validation. 3. Glutaraldehyde use: a. Read the directions for use on the bottle label and package insert. If activation is needed, pour one bottle of activator into each bottle of disinfectant, recap, and invert several times before pouring into disinfectant tube, tray or reservoir. b. Label the container with contents, hazard warnings (e.g. “Caution: Cidex Disinfectant contains glutaraldehyde, which may cause respiratory sensitization and contact dermatitis”). c. Record the date that the solution was poured from the original container, and the date that it can no longer be reused (not to exceed 14 days). Solution left in the bottle may be stored for up to 90 days. Date when opening bottle if any residual remains. 4. Test solution prior to use with Test Strips provided by manufacturer. Record test results in log. 5. Immerse clean, dry instruments completely in the glutaraldehyde or initiate reprocessing cycle. Ensure solution contacts all interior surfaces and fills lumens. 6. Cover the disinfectant tray securely and soak instruments for 20 minutes at 20 C. Automated reprocessor disinfecting cycle is 20 minutes at 25 C. 7. Manual process: Following disinfection, rinse instruments thoroughly with three rinses and dry. 8. Disinfected instruments should be used immediately, or stored in a manner to minimize recontamination. 9. After 14 days, or if the solution fails the MEC test, the glutaraldehyde solution is discarded. Follow local sanitary sewer requirements for disposal. For neutralization of HLD: a. Don personal protective equipment (chemical safety goggles, impervious long-sleeved gown, 39 decontamination gloves, shoe covers). b. Neutralize high level disinfectants in the GUS tubes or the wash/disinfection bay on the AER. Pour the correct amount of glycine neutralizer into the solution and wait for the appropriate time for neutralization to be complete. You should notice a significant color change of the solution. c. Mix slowly for 30 seconds to disperse evenly d. After the appropriate neutralization time, test the solution with a test strip to verify that it is no longer active. Log results. 10. Dispose of solution down drain and rinse basin/treatment tank with copious amounts of water. 11. Clean soaking container with soap and water before reuse. SUCCESSFUL PERFORMANCE UNSUCCESSFUL PERFORMANCE (Please Check One) Observed and Validated by: Observer/Manager Signature and Date: Employee Name Print: Employee Signature and Date: 40 KAISER PERMANENTE CIDEX OPA Disinfection Skills Validation Employee’s name (PRINT): Date: Department: □ Initial Competency □ Annual Competency Facility: Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration CRITICAL ELEMENTS Method Skill Level COMMENTS 1. Don personal protective equipment (gloves, hair cover, chemical splash goggles & fluid repellent gown). 2. Pre-soak, manually clean, rinse and dry instruments per Scope Competency skills validation. 3. OPA use: a. Read the directions for use on the bottle label and package insert. b. Label the container with contents, hazard warnings (e.g. “Caution: OPA Disinfectant contains orthophthalaldehyde, which may cause respiratory sensitization and contact dermatitis”). c. Record the date that the solution was poured from the original container, and the date that it can no longer be reused (not to exceed 14 days). Solution left in the bottle may be stored for up to 75 days. Date when opening bottle if any solution remains. 4. Test solution prior to use with Test Strips provided by manufacturer. Record test results in log. 5. Initiate reprocessing cycle. Ensure solution flows through all lumens. 6. Automated reprocessor disinfecting cycle is 12 min at 20 C or 5 min at 25 C. 7. Following disinfection cycle: don clean PPE, remove scope from AER, remove any residual moisture. 8. Disinfected instruments should be used immediately, or stored in a manner to minimize recontamination. 9. After 14 days, or if the solution fails the MEC test, the OPA solution is discarded. Disposal directly to the sanitary sewer is not permissible in California. For neutralization: Don personal protective equipment (chemical safety goggles, impervious long-sleeved gown, decontamination gloves, shoe covers). Neutralize OPA in the AER bay. Pour the correct amount of glycine neutralizer into the solution and wait for the appropriate time for neutralization to be complete. You should notice a significant color change of the 41 solution. Mix slowly for 30 seconds to disperse evenly After the appropriate neutralization time, test the solution with a test strip to verify that it is no longer active. Log results. 10. Dispose of solution down drain. Rinse AER bay with copious amounts of water and run wash cycle. SUCCESSFUL PERFORMANCE UNSUCCESSFUL PERFORMANCE (Please Check One) Observed and Validated by: Observer/Manager Signature and Date: Employee Name Print: Employee Signature and Date: 42 KAISER PERMANENTE Resert XL Disinfectant Skills Validation Employee’s name (PRINT): Date: Department: □ Initial Competency Facility: □ Annual Competency Skill Level: 1 -Novice/in training, 2 – Independent, 3 - Fully Competent/Can train others Methods of verification: O - Observation, V - Verbalization, RD Return Demonstration CRITICAL ELEMENTS Method Skill Level COMMENTS 1. Don personal protective equipment (gloves, hair cover, chemical splash goggles & fluid repellent gown). 2. Pre-soak, manually clean, rinse and dry instruments per Scope Competency skills validation. 3. Resert XL use: a. Read the directions for use on the bottle label and package insert. b. Label the AER with contents, hazard warnings (e.g. “Caution: Resert XL Disinfectant contains hydrogen peroxide, which may cause skin or respiratory tract irritation”). c. Record the date that the solution was poured from the original container, and the date that it can no longer be reused (not to exceed 21 days). 4. Test solution prior to use with Test Strips provided by manufacturer. Record test results on daily log. 5. Immerse clean, dry instruments completely in the disinfectant or initiate reprocessing cycle. Ensure solution contacts all interior surfaces and fills lumens. 6. Cover the disinfectant tray securely and soak instruments for 8 minutes at ≥ 20 C. 7. Following disinfecting, rinse instruments thoroughly with three rinses, then dry. 8. Disinfected instruments should be used immediately, or stored in a manner to minimize recontamination. Channeled scopes should be hung vertically in a closed cabinet. 9. After 21 days, or if the solution fails the MEC test, the Resert XL solution is discarded. Disposal directly to the sanitary sewer is permissible. The solution may not be used more than 21 days, even if it passes the MEC test. 10. Dispose of solution down drain and rinse AER bay with copious amounts of water. 43 SUCCESSFUL PERFORMANCE UNSUCCESSFUL PERFORMANCE (Please Check One) Observed and Validated by: Observer/Manager Signature and Date: Employee Name Print: Employee Signature and Date: \ 44 Program-wide Scope Reprocessing Scopes Reprocessing Audit Tool Date: Department: Department Manager: Facility: □ Weekly Results: □ Monthly Performance Indicator “MET” means total compliance to KP Scope Program and Recommended Practices Guidelines □ ROC Quarterly MET NOT MET 1. Reprocessing area appearance and cleanliness – floors, counters, tables, carts, high surfaces, walls, ceiling tiles, equipment, doors, floor mats. 2. Staff member recognizes scopes types, # of channels to be processed, ports, adapters, connectors. Scope manufacturer’s Instructions currently processed are available: Gastroscope Colonoscope Colonoscope, Pedi Duodenoscope/ERCP Ultrasound Scope Choledochoscope Sigmoidoscope Hysteroscope Cystoscope Ureteroscope Bronchoscope Laryngeal Intubation Rhinolaryngoscope 3. Initial and annual competencies for staff reprocessing scopes are current. 4. Proper Protective Personal Equipment is available for reprocessing and handling HLD (i.e. Nitrile, chemotherapy or butyl gloves are required when working with HLD and safety goggles when handling HLD). 5. Emergency Chemical Spill/Eye Wash/Shower within required reach and clearance. 6. Well defined workflow: Dirty to Clean (approved signage in place). 7. Proper ventilation: Air flow arm/ negative air pressure (room size/HLD volume). 8. Manual cleaning: 3 container process (wash/rinsing/disinfection) properly labeled. 9. Reprocessing Equipment - AER/Reprocessing sink/leak tester/scope cabinet in working conditions: Up to date Preventive Maintenance (filter change dates); No leaks (sink faucets and drain); scope cabinet doors closing entirely; No supplies stored under the sink/s. 10. Cleaning tools are single use, accessible and available for each type/size scope. 11. Approved cleaning and disinfecting solutions-Proper labeling of secondary containers (if applicable). Staff articulates proper dilution instructions. 12. Operators Manual for AER available. 13. Reprocessing sinks and containers for manual cleaning water level marked/labeled. 14. Visible expiration date of High-Level disinfection solution in use (i.e. on AER reservoir/ GUS unit). 15. Minimum effective concentration of high-level disinfectant is tested according to KP Scope Reprocessing Program-wide: MEC testing before each use. 16. Quality Control for HLD test strips documented (i.e. passed/failed, lot #, expiration date). 17. Activation and deactivation of HLD is properly documented (i.e. passed/failed, lot #, expiration date). (If applicable) 18. AER diagnostic test/s is performed according to KP Scope Reprocessing Programwide. 19. Transportation equipment is maintained according to KP Scope Reprocessing Program-wide. 20. Reprocessing Logs (scope reprocessing, scope cabinet cleaning, MEC testing) are regularly monitored and verified for task completion and accuracy purposes. 21. Manual and Electronic scopes reprocessing logs (AER) are reconciled with procedure cases (GI Clinic setting). 45 “MET” means the individual independently performs each responsibility MET NOT MET 22. Manual and Electronic scopes reprocessing logs reflects the required 7 elements as minimum: Patient Name Medical Record # Procedure Type Scope Serial # Scope Type Provider Name Date of Procedure Equipment use (AER/Manual) 23. Point of Use Pre-cleaning performed according to KP Scopes Reprocessing Guidelines. 24. Staff can speak to the following process: Scope Initial inspection (including scope accessories) Leak Testing: Dry and/or Wet (if applicable) Pre-cleaning: fresh cleaning solution, brushing and purging scope channels, and rinsing Scope preparation for STERRAD/ETO or High-Level Disinfection: When to Manual/Automated Disinfection: Manual final rinsing Alcohol rinse: follow scope manufacturer’s instructions Prepare for use/transportation or storage HLD label date/initials and/or expiration date 25. Process in place to identify last date scopes were processed. 26. Aseptic technique in place to remove scope from scope cabinet. 27. Process in place to properly transport scopes from/to reprocessing area. 28. Process in place to identify, process and document scopes going to/from repair. 29. Defined equipment preventive maintenance program in place. 30. In-service/training provided for every new scope type in inventory. Findings Recommendations Audit performed by: Signature and Date: Audit findings received by: Signature and Date: Actions 46 11. POST TEST KAISER PERMANENTE Program-wide Scope Reprocessing POST TEST Scope Reprocessing Employee’s name (PRINT): Dept/Facility: Results Score: Date: Multiple choice: Circle letter of each answer 1. What universal/standard precautions should be followed by those reprocessing scopes? a) wear nitrile, chemotherapy or butyl gloves b) wear impervious gown c) wear mask, eye protection such as goggles d) all of the above. 2. What is the first thing you need to do with the scope following the procedure? a) wipe the scope exterior down with an endoscope compatible detergent solution b) submerge entire scope in compatible detergent solution c) brush channel 3. Following wiping down the scope with an endoscope compatible detergent solution and suctioning solution through channels, what is the next process? a)clear air/water channels according to the manufacturer b) submersing scope in high level disinfectant c) drying scope with alcohol and air. 4. The most important step after clearing the air/water channels of the scope, in preventive maintenance of the flexible scope is: a) drying the scope b) brushing the scope c) leak testing the scope 5. What parts of the scope should be submerged for leak testing? a) distal tip only b) distal tip, followed by the insertion tube; c) the entire scope 6. Point of Use Pre-cleaning, leak testing & manual cleaning of the scope must be done before submersing the scope in high level disinfectant. a) yes b) no 7. After cleaning the scope with brushes and other cleaning devices, what procedure should be used for cleaning channels? a) flush with tap water and purge with air b) flush with compatible detergent solution followed by tap water & purge with air c) manual: fill channels with compatible detergent solution & allow to soak; flush with tap water and purge with air automated: use compatible detergent solution. 8. What step comes after Point-of-Use Precleaning the scope: a) leak testing b) high level disinfecting the scope c) fill with compatible detergent, rinse with tap water, & purge with air. 9. According to the SGNA guidelines, if proper Point-of-Use Pre-cleaning has taken place, what is the proper amount of time the scope must be submerged in high level disinfectant? a) Glutaraldehyde for 30 minutes; OPA for 12 minutes (5 minutes if automated) b) Glutaraldehyde for 20 minutes; OPA for 12 minutes. (5 minutes if automated) 10. Following high level disinfection, what is the next step? a) putting scope in storage cabinet b) rinsing the scope with sterile water; or tap water followed by rinsing with 70% alcohol (manual disinfection: three water rinses – exterior of scope plus 100 ml each lumen/each rinse; automated disinfection: with AER default cycle and rinse times) c) drying the scope 11. Following the final rinse phase after every tap water rinse and prior to storage after sterile water rinsing, a 70% alcohol flush is required. The main purpose for the alcohol flush is? a) to ensure rinsing is thorough 47 b) kill any remaining microorganisms c) facilitate drying 12. What is the proper method for reprocessing reusable biopsy forceps? a) clean with compatible detergent solution, use brush on tips to clean, rinse, ultrasonically clean, then steam sterilize b) clean with compatible detergent solution, use brush on tips to clean, rinse, ultrasonically clean, then high level disinfect c) clean with compatible detergent solution, use brush on tips to clean, rinse, ultrasonically clean, then EtO (sterilize). 13. Should biopsy forceps be reprocessed with flexible scopes in the same container? a) yes b) no 14. With what frequency should high level disinfectant solution be tested for effective concentration? a) daily if disinfectant does not fail early b) prior to each use c) both A & B 15. What is one of the most important considerations when using the Automated Reprocessor? a) establishment of correct connectors between the AER and the scope is critical to ensure complete flow of disinfectants and rinse water b) ensure that two rinses are performed by the processor (for all scopes) and ensure flow through all ports during alcohol rinse step (for channeled scopes) c) set the machine for the recommended time 16. Which Sterrad NX sterilizer statement is NOT true: a) only a single channeled flexible scope no more that 850 cm in length and 1 mm in diameter or greater may be sterilized in the Sterrad NX b) the device must be thoroughly dry or the cycle will abort c) the flexible scope may be run on either the standard or the advanced cycle d) Tyvek pouches are the only pouches validated for use in the Sterrad NX 17. After its empty, the Sterrad cassette should be placed in a biohazardous waste receptacle. a) true b) false Employee Signature: _______________________________________________Date________________________ Auditor Signature: _________________________________________________Date_______________________ Manager Signature: ________________________________________________Date_______________________ 48 12. POST TEST – Manager ANSWER KEY KAISER PERMANENTE Program-wide Scope Reprocessing POST TEST – ANSWER KEY Multiple Choice: 1. What universal/standard precautions should be followed by those reprocessing scopes? d. All of the above 2. What is the first thing you need to do with the scope following the procedure? a. Wipe the exterior scope down with a compatible detergent. 3. Following wiping down the scope with enzymatic compatible detergent and suctioning compatible detergent through channels, what is the next process? a. Clear air/water channels according to the manufacturer 4. The most important step after cleaning the air/water channels of the scope, in preventive maintenance of the flexible scope is: c. Leak testing the scope 5. What parts of the scope should be submerged for leak testing? c. The entire scope 6. Point of Use Pre-cleaning, leak testing & manual cleaning of the scope must be done before submersing the scope in high level disinfectant. a. Yes 7. After cleaning the scope with brushes, sponges, and other cleaning devices, what procedure should be used for cleaning channels? c. manual: fill channels with compatible detergent & allow to soak; flush with tap water and purge with air; automated: use compatible detergent 8. What step comes after Point of Use Pre-cleaning the scope? a. Leak testing the scope 9. According to the SGNA guidelines, if proper Point of Use Pre-cleaning has taken place, what is the proper amount of time the scope must be submerged in high level disinfectant? b. Glutaraldehyde for 20 minutes; OPA for 12 minutes (5 minutes if automated). 10. Following high level disinfection, what is the next step? b. Rinsing the scope with sterile water, or tap water followed by 70% alcohol rinse. Rinsing should be done three times after manual disinfection – exterior plus 100 mL water per lumen/per rinse; and two times after automated disinfection – interior and exterior with four minute cycles. All rinsing should be followed by forced air-drying. Sterile water must be discarded after use. 11. Prior to storage, and after tap water rinsing, a 70% alcohol flush is required. The main purpose for the alcohol flush is: c. Facilitate drying 12. What is the proper method for reprocessing reusable biopsy forceps? a. Clean with compatible detergent, use brush on tips to clean, ultrasonically clean, rinse, then steam sterilizes. 13. Should biopsy forceps be reprocessed with flexible scopes in the same container? b. No 14. With what frequency should high level disinfectant solution be tested for effective concentration? b. Prior to each use 15. What is the most important consideration when using an automated reprocessor? a. Establishment of correct connectors between the AER and the scope is critical to ensure complete flow of disinfectants and rinse water 16. Which Sterrad NX sterilizer statement is NOT True: c. The flexible scope may be run on either the standard cycle or the advanced cycle 17. After it is empty, the Sterrad cassette should be placed in a biohazardous waste receptacle a. True 49 13. Documentation of Employee Completion of Competency package KAISER PERMANENTE Program-wide Scope Reprocessing DOCUMENTATION FORM EMPLOYEE NAME: DATE: DEPT: OBSERVER/TRAINER NAME: In order to ensure competency in scope reprocessing, each staff person should accomplish the steps below. This should be done at the time of hire and annually thereafter to demonstrate competency according to the 1999 FDA Recommendations. Please indicate completion of scope reprocessing competency package by placing a check next to each item below, once completed by employee: a) Review Scope Reprocessing Policy and Procedure and Quick Reference Grid b) Review the APIC Guidelines for Infection Prevention and Control in Flexible Endoscopy; American Journal of Infection Control, 1999; 28:138-155 or comparable information. c) Review the SGNA Chart: Steps Necessary to Thoroughly Clean and High Level Disinfect Flexible Endoscopes; Society of Gastroenterology Nurses and Associates, Inc – 5th Edition 2009 or comparable information. 4. Olympus Scope Video: http://www.olympusamerica.com/msg_section/cds/cds_position.asp 5. Staff must provide return demonstration for all reprocessing tasks including: Completing Competency Tool with observer/trainer and successfully performing all reprocessing tasks in compliance with Medical Center Policies and Procedures, including: PPE: Personal Protective equipment Point of Use Pre-cleaning Leak Test Cleaning Disinfection Rinsing/Drying Sterilization Accessories Storage Appropriate Preventative Maintenance and Record Keeping 4. Complete Proof of Competency by: Completing post test and reviewing answers with observer/trainer 50 14. Definitions AER Automated endoscope reprocessor Antiseptic A chemical germicide formulated for use on skin or tissue and should not be used to decontaminate inanimate objects Bactericide An agent that kills bacteria Cleaning The removal of all foreign material (e.g., soil, organic material) from objects Contaminated The presence of pathogenic organisms on or in an inanimate object Critical Term used to a describe a category for items that will come in contact with sterile tissue Decontamination The process of removing disease-producing microorganisms on a surface or item Disinfectant A germicide that inactivates virtually all recognized pathogenic microorganisms but not necessarily all microbial forms (e.g., bacterial endospores) on inanimate objects Germicide An agent that destroys microorganisms, particularly pathogenic microorganisms High-level disinfection Term used for the process of killing all microorganisms, with the exception of high numbers of bacterial spores; Abbreviation = HLD Intermediate-level disinfection Term used for the process of killing Mycobacterium tuberculosis, vegetative bacteria, most viruses, and most fungi, but does not necessarily kill bacterial endospores Low-level disinfection Term used for the process of killing most bacteria, some viruses, and some fungi, but it cannot be relied on to kill tubercle bacilli or bacterial endospores Minimum effective concentration Refers to the lowest concentration of active ingredient necessary to meet the label claim of a reusable high-level disinfectant/sterilant Non-critical Term used to describe a category for items that may come in contact with intact skin but not with mucous membranes Semi-critical Term used to describe a category for items that will come in contact with mucous membranes or nonintact skin. Sterilization The complete elimination or destruction of all forms of microbial life 51 15. Algorithm of Investigation for Potential Exposure Related to Scope Reprocessing Identification of potential patient exposure associated with scope reprocessing Request IC/ID review available data to confirm and quantify potential patient exposure IC/ID to contact CDC and/or other content expert for assistance as needed in exposure determination: Share initial findings/recommendations with key internal decision makers and stakeholders (e.g. Leadership, Risk, Operations.) Convene investigative team and notify local DHS and local SMT, if appropriate YES Patient notification appropriate? YES Initiate chart reviews, interviews, observational process and clinical investigation by designated team Identify and recommend appropriate control and exposure follow-up measures Notify patients by phone and letter Complete final report, follow lab results to determine if associated infections. Establish monitoring process to assure continuation of control measures Notify Regional IC and Public Affairs Obtain feedback and recommendations for corrective actions NO Staff education and monitoring indicated SAMPLE - Scope Inventory – Name of Hospital Initial competency requires each scope to be reprocessed correctly 3 times. Annual competency requires each scope to be processed correctly once unless otherwise requested by manager. Scope/Mfg. Employee Signature Date Employee Signature Date Employee Signature Date Proctor Signature Date Proctor Signature Date Proctor Signature Date Employee Signature Date Employee Signature Date Employee Signature Date Proctor Signature Date Proctor Signature Date Proctor Signature Date Employee Signature Date Employee Signature Date Employee Signature Date Proctor Signature Date Proctor Signature Date Proctor Signature Date Employee Signature Date Employee Signature Date Employee Signature Date Date URF P 3 Olympus CYF TYPE 2T 160L Olympus CYF 5 Olympus GIF TYPE Q 180 Olympus Proctor Signature Date Proctor Signature Date Proctor Signature Employee Signature Date Employee Signature Date Employee Signature Date Proctor Signature Date Proctor Signature Date Proctor Signature Date Employee Signature Date Employee Signature Date Employee Signature Date Proctor Signature Date Proctor Signature Date Proctor Signature Date Employee Signature Date Employee Signature Date Employee Signature Date Proctor Signature Date Proctor Signature Date Proctor Signature Date GIF TYPE 2T 160 Olympus HYF TYPE V Olympus PCF TYPE H 180AL Olympus