1 Instruction for medical application of the preparation of Diclofenac
advertisement

1 Instruction for medical application of the preparation of Diclofenac-AKOS Trade name: Diclofenac -AKOS International nonproprietary name: Diclofenac Dosage form: solution for intramuscular injection Composition: Active substance: diclofenac sodium -25 mg. Accessory substances: benzyl alcohol, propylene glycol, water for injection – up to 1 milliliter. Description: transparent slightly tinted liquid with slight smell of benzyl alcohol. Pharmacotherapeutic group: Non steroidal anti-inflammatory drug (NSAID). ATC-code: [M01AB05] Pharmacological action Diclofenac possesses anti-inflammatory, anaesthetic and antipyretic action. Nonselectively depressing cyclooxygenase 1 and 2, it disturbs metabolism of arachidonic acid, decreases the quantity of prostaglandins in an inflammatory tissue. In case of rheumatic deseases the anti-inflammatory and analgesic effect of diclofenac promotes considerable reduction of intensity of pain, morning morning woodiness, arthrocele, so it improves the functional status of the movable joint. In traumas, in a post-operation period the diclofenac reduces pain sense and inflammatory edema. Pharmacokinetics The time of achievement of the maximum concentration in intramuscular application in dosage of 75 milligrams is 15-30 minutes, value of maximum concentration is 1.9-4.8 (on the average 2.7) µg/milliliter. In 3 hours after injection the plasma concentration averages 10 % from the maximum value. Connection with plasma proteins is more than 99 % (the most portion is bound with albumins). Metabolism results from multiple or single hydroxylation and conjugation with glucuronic acid. Р450 CYP2C9 enzyme system takes part in metabolism of the preparation. The pharmacological activity of the metabolites is lower than that of diclofenac. 2 The systemic clearance is 350 ml/minute, the volume of distribution is 550 ml/kg. The plasma elimination half-life is 2 hours. 65 % of the given dose is egested in the form of metabolites by the kidneys; less than 1% is egested in an unaltered form, the remaining portion of the dosage is egested in the form of metabolites with bile. In patients with frank renal insufficiency (the creatine clearance is less than 10 ml/minute the egestion of metabolites with bile is increased, at that increase of their concentration in the blood stream is not observed. In patient with chronic hepatitis or compensated liver cirrhosis pharmacokinetic properties of diclofenac do not alter. Diclofenac penetrates with human milk. Indications to application For short-time treatment of pains of various genesis, moderate intensity: - diseases of locomotor apparatus (rheumatic arthritis, psoriasic, juvenile chronic arthritis, ankylosing spondylitis; gouty arthritis, rheumatic soft tissue involvement, osteoarthrosis of peripheral movable joints and spinal column (including with radicular syndrome). The preparation is intended to be applied for symptom management, decrease of pain and inflammation on the moment of usage, it does not affect advance of the disease; - lumbago, ischias, neuralgia; - algodismenorrhea, inflammatory process of the organs of small pelvis including adnexitis; - posttraumatic pain syndrome accompanied by inflammation; - postoperative pain. Contra indications Hyperresponsiveness (including to other NSAIDs or auxiliary components), erosiveulcerative damage of gastrointestinal tract (in-phase of aggravation), issue of blood out of gastrointestinal tract, inflammatory bowel diseases (non specific ulcerative colitis, Crohn's disease), frank hepatic insufficiency, liver diseases in acuity, frank renal insufficiency (creatine clearance is less than 30 ml/minute), cardiac insufficiency, hyperkalemia, complete or incomplete syndrome of intolerance of acetosalicylic acid (rhinosinusitis, urticaria fever, polypus of nose mucosal, bronchial asthma, appearing in administration of acetosalicylic acid or other NSAIDs), dyshematopoiesis, abnormalityof hemostasis (including 3 haemophilia), pregnancy, childhood (under 18 years) , lactation period, period after conduction of aortocoronary shunt procedures. With care: ischemic heart disease, cerebrovascular disease, backward heart failure, arterial hypertension, heavy drop of the volume of blood circulation (including after massive surgical intervention), edema syndrome, diseases of peripheral arteries, dislipidemy, pancreatic diabetes, anaemia, bronchial asthma, renal insufficiency (creatine clearance is less than 60 ml/ minute), alcoholism, erosive-ulcerative diseases of gastrointestinal tract outside of aggravation, diseases of liver in anamnesis, diverticulitis, status post vast surgical intervention, induced porphyria, elderly age, smoking, severe somatic diseases, systemic diseases of connective tissue, long-term use of nonsteroidal antiinflammatory drugs, simultaneous administration of glucocorticosteroids, anticoagulating agents, antiaggregants, selective dissociable inhibitors of serotonin reuptake, hyperlipidemia, presence of infection of Helicobacter pylori. Mode of administration and dosage It should be injected deep intramuscular. A single dose for adults is 75 milligrams (1 ampoule). As required a repeated administration is possible, but not earlier than in 12 hours. With the use of other dosage forms of diclofenac the maximum daily dose of 150 milligrams should not be exceed. The duration of the usage is at most 2 days, if the further duration is necessary then transfer to peroral or rectal application of diclofenac. Side effects For decrease of risk of development of undesirable occurrences one should use minimum effective dose for a short course. Often - 1-10 %; sometimes – 0.1-1 %; seldom – 0.01-0.1 %; very seldom – less than 0.001 %, including individual cases. On the part of digestive system: - often - epigastric pain, nausea, vomit, diarrhea, dyspepsia, meteorism, anorexia, increment in activity of aminotransferases; - seldom - gastritis, proctitis, issue of blood out of gastrointestinal tract (vomit with blood, melanorrhagia, diarrhea with bloody tap), ulcers of gastrointestinal tract (with or without issue of blood or perforation), hepatitis, jaundice, compromised liver function; - very seldom - stomatitis, glossitis, damage of esophagus, diaphragm like stricture of intes- 4 tinal canal (heterospecific haemorrhagic colitis, aggravation of ulcerative colitis or Crohn's disease), astriction, pancreatitis, fulminant hepatitis. On the part of nervous system: - often - headache, paraequilibrium; - seldom - drowsiness; - very seldom - abnormality of sensibility including paresthesia, memory corporals, tremor, convulsion, alarm, cerebrovascular abnormality, aseptic meningitis, disorientation, depression, insomnia, paroniria, irritancy, psychiatric disturbances. On the part of sense organs: - often - vertigo; - very seldom - impairment of vision , hearing disorder, tympanophonia, abnormality of taste sense modality. On the part of urinary system: - very seldom - acute renal failure, hematocyturia, proteinuria, interstitial nephritis, nephrotic syndrome, papilliferous necrosis. On the part of blood-making organs: - very seldom - thrombocytopenia, leukopenia, globulicidal and atrophic anemia, agranulocytosis Hypersensitivity reaction: - anaphylactic/anaphylactoid reactions including frank decrease of arterial tension and collapse; - very seldom - angioneurotic edema (including of the face). On the part of cardiovascular system: - very seldom - heartbeats, stethalgia, arterial tension rise, vasculitis, cardiac insufficiency, myocardial infarction. On the part of respiratory system: - seldom - bronchial asthma (including labored breathing); - very seldom - pneumonitis. On the part of skin integument: - often - exanthema; - seldom - urticaria fever; 5 - very seldom - bullous eruption, eczema including multiform and Stevens-Johnson syndrome, Lyell's syndrome, exfoliative dermatitis, itch, epilation, photoallergy, purpura including allergenic. Local reactions in intramuscular injection: - burning, infiltrate, simple necrosis, necrosis of fatty tissue. Overdosage Symptoms: vomit, paraequilibrium, headache, labored breathing, turbidity consciousness, in childrens - myoclonus, nausea, vomit, abdominal pain, issue of blood, compromised liver and kidney function. Treatment: symptom management, artificial diuresis. Haemodialysis is inefficient. Interaction with other medicinal preparations It raises concentration of digoxin, methotrexate, lithium preparations and cyclosporine in the blood plasm. It decreases effect of diuretic, against the background of potassium-sparing diuretics increases risk of hyperkalemia; against the background of anticoagulating agents, thrombolytic agents (alteplase, streptokinase, urokinase) there is a risk of issue of blood (more often out of the gastrointestinal tract). It reduces effects of hypotensive and hypnotic agents. It increases probability of rise of adverse events of other nonsteroidal anti-inflammatory preparations and glucocorticosteroid remedies (issue of blood in the gastrointestinal tract), toxicity of methotrexate and nephrotoxicity of cyclosporine. Acetosalicylic acid decreases concentration of diclofenac in blood. Simultaneous use with paracetamol enhances risk of development of nephrotoxic effects of diclofenac. It reduces effect of hypoglycemic agents. Cefamandole, cefoperazone, cefotetan, valproic acid and plicamycin increase frequency of development of hypoprothrombinemia. Cyclosporine and gold preparations enhance impact of diclofenac on synthetic process of prostaglandins in kidneys, that enhances nephrotoxicity. Simultaneous prescription with ethanol, colchicine, adrenocorticotropic hormone, selective dissociable inhibitors of serotonin reuptake, and preparations of goatweed enhances risk of development of issue of blood in the gastrointestinal tract. 6 Diclofenac strengthens action of the preparation inducing photoallergy. Preparation blocking canalicular release raise diclofenac concentration in the blood plasm, therefore enhancing its toxicity. Antibacterial medicinal preparations of the group quinolones have risk of convulsion development. Special instructions The patients using the preparation should abstain from kinds of activity requiring increased attention and quick psychic and motor responses, alcohol consumption. Product form Solution for intramuscular injection of 25 milligrams/milliliter. Ampoule of neutral glass of 5 milliliter capacity contain 3 milliliters. There are 5 or 10 ampoules in a cardboard box. There are 5 ampoules in a contour cellular packaging of polyvinylchloride film and aluminium printed lacquered foil, or without foil. There are 1, 2 contour cellular packagings in a cardboard package. Each package and box have the application instructions and ampoule scarificator enclosed. In case of usage of the ampoules with ring of fracture or notched and having a point the scarificator is not enclosed. Shelf life 2 years. Do not use after expiry date. Storage conditions List B. It should be stored in a place protected from light at temperature from 15 to 25° C. Freezing is not allowed. Keep out of reach of children. Pharmacy purchasing terms: Prescription medicine. Manufacturer/Organization accepting customer complaints: Open Joint Stock "Kurgan Joint Stock Company of Medical Preparations and Articles "Sintez" (Sintez JSC No. 7, Prospect Konstitutsii, city of Kurgan, Russian Federation, 640008 Tel/fax (3522) 48-16-89 e-mail: real@kurgansintez.ru , Web site of the manufacturing company: http://www.kurgansintez.ru



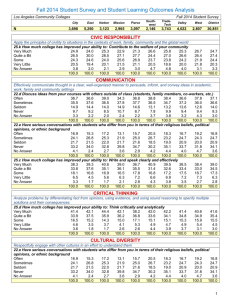



![to Learning Styles Questionnaire [MS Word,93Kb]](http://s3.studylib.net/store/data/007287401_2-741c6340dee171d22423967f2d0c2716-300x300.png)

