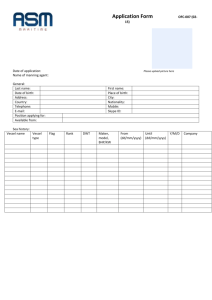
Purified water- water obtained by deionization, distillation, ion
advertisement

Q: Do you have to use sterile water to make amiodarone oral liquid suspension with methylcellulose and simple syrup or can tap or purified water be used? Also do products containing water require a 14-day expiry date? The evidence to answer this question is very limited. As noted by Nahata et al. many pediatric medications are made by compounding but specific information regarding these compounds is not available. (1) After having reviewed tertiary and primacy literature the following was found: Tap water cannot be used to make oral suspensions as tap water contains salts such as calcium, aluminum, iron etc. (2) The calcium can change the viscosity and strength of gels made of algins and pectins and can compromise the clarity and quality of some mixtures. (2) Purified water is different from tap water as it is made by deionization, distillation, ion-exchange, reverse osmosis, filtration and other suitable procedures. (2, 3) Although the water has had the salts removed, the bacterial content of the purified water is unknown, as it depends on the purification process used, so it cannot be used for sterile purposes or when bacterial growth is a concern. (2) The evidence for expiry dates is conflicting. (1, 4) Nahata et al. states that all medications without specific stability information must have a 14-day expiry. (1) Allen says that for water containing products a 14-day expiry is normally used and for all other products without water a 30-day expiry is needed. (1, 4) In addition, if there is a specific study that proves a longer duration of stability of a water or non-water containing product then the studied formulation and expiry date can be used. (4) For example, the formulation found in Annals of Pharmacotherapy has a 91 day expiry even though the amiodarone suspension contains water. (4, 5) In the Annals of pharmacotherapy, a study of the stability of amiodarone in an oral suspension showed that amiodarone is stable for 91 days at 4 degrees Celsius and 42 days at room temperature (25 degrees Celsius). (5) This compounding formula used amiodarone tablets dissolved in purified water prepared in methylcellulose 1% and syrup. In this study they measured the change in pH and concentration and neither were affected over the 91 days. (5) With this specific formulation sterile water was not used; however, information regarding general compounding and the use of sterile water versus purified water is not available; therefore, the safest option (sterile water) or the type of water specified in the formula should be used. (5) 1) Nahata MC, Allen LV. Extemporaneous drug formulations. Clinical Therapeutics 2008 Nov 11; 30:2112-18. 2) Wade A, Weller PJ. Editor. Handbook of pharmaceutical excipients. Washington: American Pharmaceutical Association; 1994.p.548 3) Troy DB. Editor. Remington: The Science and Practice of Pharmacy. Philadelphia: Lippincott Williams & Wilkins; 2005. P.746. 4)Allen LV. Allen’s compounding formulations. Washington: American Pharmaceutical Association; 2003.p.3. 5) Nahata MC. Stability of Amiodarone in An oral suspension. Ann Pharm 1997 July/Aug;31(7/8):851-55.