Polymers
advertisement

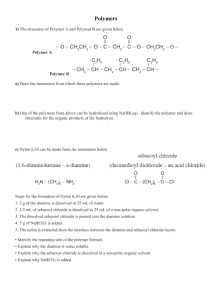
Polymers Polymers are an extremely important material in our world. Plastics are the most commonly used polymer, and they are all around us, intertwined in our daily lives. How many of you brushed your teeth this morning? Well, that was not horsehair on your toothbrush! (I hope!) How many of you put on clothes? Hopefully all of you. Ever paid attention to that material label inside your shirt or pants? Anyone wearing Nylon or Dacron or polyester today? Because of their abundance in our society, as chemists, we should study the properties as well as structures of polymer materials. Polymers are extremely large molecules with molar masses exceeding 1.0 x 106 grams/mole. A polymer is a material composed of many repeating units called monomers. Monomers are molecules which have the structural ability to react and form a repeating sequence. The chemical structure of a monomer affects both the type of polymer that it can form as well as the way in which the polymer forms in a chemical reaction. The main classes of polymer materials are: addition polymers and condensation polymers. An addition polymer is formed from a compound that contains a multiple bond. An initiator is used to open the first monomer's multiple bond, but once the reaction starts, the open monomer reacts with other monomers in order to form a polymer. This reaction requires an initiator, like benzoyl peroxide or t-butyl benzoyl peroxide. Initiators are unstable molecules that split apart into free radicals. Free radicals are highly reactive! They cause the first monomer’s double bond to open and allow the polymerization process to begin. Often heat or ultraviolet light are needed before the initiator will form free radicals; however, initiators (especially, peroxide initiators) should be handled with care because they can explode if dropped with sufficient force. Polymers all share some similar properties. These include: 1) the flexibility and structure 2) strength (try ripping apart your nylon coat!) 3) the presence of crosslinks (usually covalent bonds) between polymer chains Crosslinking can occur if there is a double bond or other reactive group present on a side chain. These reactive areas can be used to create new bonding regions which will make a stronger, more rigid material. Crosslinking restricts the ability of the individual polymer chains to slide past one another. Crosslinking can be thought of as a three-dimensional network structure. Depending on the degree of crosslinking that occurs, different properties result. Low levels of crosslinking, such as elastomers (e.g. rubber bands) are elastic and deformable. Highly crosslinked materials, such as Bakelite (billiard balls) are obviously rigid and brittle. 1 Because we use so much plastic, recycling is an excellent way to conserve resources. A code for plastics was established based on the most frequently used plastics (see chart below). Methyl Methacrylate: DANGER! HEATING, SUNLIGHT OR CONTACT WITH INCOMPATIBLE MATERIALS MAY CAUSE EXPLOSIVE POLYMERIZATION. FLAMMABLE LIQUID AND VAPOR. HARMFUL IF SWALLOWED OR INHALED. AFFECTS CENTRAL NERVOUS SYSTEM. CAUSES IRRITATION TO SKIN, EYES AND RESPIRATORY TRACT. MAY CAUSE ALLERGIC SKIN REACTION. MAY BE HARMFUL IF ABSORBED THROUGH SKIN. Poly(methyl methacrylate), which lazy scientists call PMMA, is a clear plastic, used as a shatterproof replacement for glass. The barrier at the ice rink which keeps hockey pucks from flying in the faces of fans is made of PMMA. The chemical company Rohm and Haas makes windows out of it and calls it Plexiglas. Ineos Acrylics also makes it and calls it Lucite. Lucite is used to make the surfaces of hot tubs, sinks, and the ever popular one piece bathtub and shower units, among other things. PMMA is a member of a family of polymers which chemists call acrylates, but the rest of the world calls acrylics. Another polymer used as an unbreakable glass substitute is polycarbonate, but PMMA is cheaper! When it comes to making windows, PMMA has another advantage over glass. PMMA is more transparent than glass. When glass windows are made too thick, they become difficult to see through. But PMMA windows can be made as much as 13 inches (33 cm) thick, and they're still perfectly transparent. This makes PMMA a wonderful material for making large aquariums, whose windows must be thick in order to contain the high pressure millions of gallons of water. In fact, the largest single window in the world, an observation window at California's Monterrey Bay Aquarium, is made of one big piece of PMMA which is 54 feet long, 18 feet high, and 13 inches thick (16.6 m long, 5.5 m high, and 33 cm thick). PMMA is also found in paint. The painting on your right, Acrylic Elf was painted by Pete Halverson with acrylic paints. Acrylic "latex" paints often contain PMMA suspended in water. PMMA doesn't dissolve in water, so dispersing PMMA in water requires we use another polymer to make water and PMMA compatible with each other. But PMMA is more than just plastic and paint. Often lubricating oils and hydraulic fluids tend to get really viscous and even gummy when they get really cold. This is a real pain if you trying to operate heavy equipment in really cold weather. When a small amount of PMMA is dissolved in these oils and fluids, they do not get viscous in the cold, and machines can be 2 operated down to -100 oC! (the question is, can the human operating the machine take that weather!!) PMMA is made by free radical vinyl polymerization from the monomer methyl methacrylate. As you can see from the picture, the structure of methyl methacrylate kind of looks like Massachusetts. But Massachusetts does not polymerize, because there is only one. NYLON 6,6: Nylons are some of the most important fibers produced commercially. If you have ever slept in a tent or used a toothbrush, you have used nylon fibers. But nylon can be more than just fibers. It is also used for self-lubricating gears and bearings. Nylon-clay composites are used to make under-hood automobile parts. The two most important kinds of nylon are nylon 6,6 and nylon 6. These two nylons have almost identical properties. Both were invented in the late 1930s. Nylon 6,6 was discovered first. It was invented in the United States by Wallace Carothers who was working for DuPont. Not long after that Nylon 6 was invented in Germany by Paul Schlack who was working for I.G. Farben. Physical Properties You may ask yourself, "Why does nylon act as it does?" You may ask yourself, "Why does nylon make such good fibers?" The answer to both is pretty simple: intermolecular forces. When you are talking about nylons, the most important intermolecular force is hydrogen bonding. The nitrogen-bonded hydrogens of one nylon chain will hydrogen bond very strongly with the carbonyl oxygens of another nylon chain. These hydrogen bonds make crystals of nylon very strong, because they hold the nylon chains together very tightly. Of course, these strong crystals make strong fibers. Unless it has been drawn into fibers, only about 20-30% of the nylon in a given sample is crystalline when in solid form. The rest is in the amorphous phase. But even though it's non-crystalline, the chains are still bound strongly to each other by hydrogen bonds. This combination of crystalline and strongly associated amorphous phases is what makes nylon thermoplastics so tough. (This only applies to nylons used as thermoplastics, mind you. When drawn into fibers nylons become almost entirely crystalline). 3 We all know that a lot of the nylon produced ends up as clothing. But it also ends up as other everyday things like rope, tents, and toothbrush bristles. Sometimes nylon is used to make the belts that reinforce tires. Most passenger car tires have steel belts, but tires for aircraft, trucks and off-road vehicles are often made of nylon. Under the hood of your car you'll find nylon fibers reinforcing rubber belts, too. Theory Step-growth Polymerizations and Chain-growth Polymerizations All polymerizations fall into two categories: step-growth polymerizations and chain-growth polymerizations. Both step-growth polymerizations and chain-growth polymerizations are used to make nylons. Making nylon from a diacid and a diamine is a step-growth polymerization. So what is the difference between the two types of polymerization? There are some practical differences you should know about for this experiment... In a step-growth system, we start off with monomers. The monomers combine and grow into dimers, trimers, tetramers, and so forth. The molecules get bigger and bigger, but only when you done (when the polymerization reaches high conversion) do you have high molecular weight polymers. But in a chain growth system, again you start off with monomers, but the monomers quickly form high molecular weight polymers. There are high molecular weight polymers present in your test tube just after you start the polymerization. There are no dimers, trimers, and other oligomers (many units) hanging around. A growing polymer chain grows so fast that it reaches high molecular weight quickly, and it does not spend any real length of time as an oligomer. Polycondensations There is another way to describe polymerizations other than the step-growth/chain-growth system. There is also the condensation/addition system. The most important thing you need to know about this system is that it classifies all polymerizations as polycondensations or polyadditions. Polycondensations are polymerizations in which a small molecule by-product is produced. The by-product is usually something like water, HCl, or once in awhile NaCl. Polyadditions on the other hand are polymerizations in which no by-product is produced. 4 Polycondensation can be used to make nylons. The simplest polycondensation for making nylons is the polymerization of a diacid and a diamine. This reaction might not normally go to high conversions (make a lot of nylon as the product), but by removing the water by-product, we can force this reaction go to higher conversions. Removing water makes the reaction go to high conversion thanks to LeChatlier's Principle. Our preparation of nylon uses the diacid, and is a condensation polymer. It is produced when a diamine and a diacid chloride react. The small molecule produced is hydrochloric acid. Today in lab, you will react 1,6-hexanediamine and adipoyl chloride to form nylon-6,6 and HCl. The structures for the monomers and polymer are shown below. The Silly Polymer: Silly Putty is a dilatant compound, a silicone based polymer that is highly elastic, exhibits high bounce, can be easily molded, yet can hold it shape while at rest. It is non-toxic and non-irritating to the skin. The history of silly putty is quite amusing. In 1943 James Wright, an engineer, was attempting to create a synthetic rubber. He was unable to achieve the properties he was looking for and put his creation (later to be called silly putty) on the shelf as a failure. A few years later, a salesman for the Dow Corning Corporation was using the putty to entertain some customers. One of his customers became intrigued with the putty and saw that it had potential as a new toy. In 1957, after being endorsed on the "Howdy Doody Show", silly putty became a toy fad. Recently new uses such as a grip strengthener and as an art medium have been developed. Silly putt even went into space on the Apollo 8 mission. 5 The polymers in silly putty have covalent bonds within the molecules, but hydrogen bonds between the molecules. The hydrogen bonds are easily broken. When small amounts of stress are slowly applied to the putty, only a few bonds are broken and the putty "flows". When larger amounts of stress are applied quickly, there are many hydrogen bonds that break, causing the putty to break or tear. If a substance springs back to its original shape after being twisted, pulled, or compressed, it is most likely a type of polymer called an elastomer. The elastomer has elastic properties (i.e., it will recover its original size and shape after being deformed). An example of an elastomer is a rubber band or a car tire. The liquid latex (Elmer's glue) which you use contains small globules of hydrocarbons suspended in water. The silly putty is formed by joining the globules using sodium borate (a cross-linker). The silly putty is held together by very weak intermolecular bonds that provide flexibility around the bond and rotation about the chain of the cross-linked polymer. If the cross-linked bonds in a polymer are permanent, it is a thermosetting plastic. If the bonds are non-permanent, it can be considered either thermoplastic or an elastomer. The covalent bonds along the chain are strong, but the bonds between chains are normally weak. However, additives such as borax allow the formation of strong "cross-links" between chains, such as CB-C. As the number of cross-links increases, the material becomes more rigid and strong. Borax crosslinking in a polymer I’m Melting . . . Polystyrene is an inexpensive and hard plastic, and probably only polyethylene is more common in your everyday life. The outside housing of the computer you are using now is probably made of polystyrene. Model cars and airplanes are made from polystyrene, and it also is made in the form of foam packaging and insulation. Clear plastic drinking cups are made of polystyrene, so are a lot of the molded parts on the inside of your car, like the radio knobs. Polystyrene is also used in toys, and the housings of things like hairdryers, computers, and kitchen appliances. What we commonly call styrofoam, is actually the most recognizable form of foam polystyrene packaging. Styrofoam ® is a Dow Chemical Co. trademarked form of polystyrene foam insulation, introduced in the U.S. in 1954. Styrofoam® is a trademarked name, the real name of the product is foamed polystyrene. 6 Polystyrene is a vinyl polymer. Structurally, it is a long hydrocarbon chain, with a phenyl group attached to every other carbon atom. Polystyrene is produced by free radical vinyl polymerization, from the monomer styrene. Polystyrene is a polymer which is cross-linked to provide extra stability in keeping its shape. Air (or other common gas) can be blown into molten polystyrene (plastic) to create a light and foamy material called styrofoam. This air-filled product is good for packing and insulation. Water tends to dissolve molecules that contain OH groups (hydrophilic), but styrofoam does not have OH groups associated with them which makes styrofoam cups good to drink out of! However, styrofoam cups cannot hold all types of liquid, as you shall soon see! Procedure: I. Preparation of Polymethyl Methacrylate (Lucite, PMMA) a. Add 5 mL of methyl methacrylate to a clean, dry, DISPOSABLE test tube b. Add about 0.025 grams of benzoyl peroxide to the test tube c. Place a rubber stopper in the test tube and swirl (not bartender shake) the test tube to dissolve the benzoyl peroxide d. Remove the rubber stopper and place the test tube in a beaker of gently boiling water – do not smell/inhale any vapors – let’s open the doors! e. Watch the test tube closely. When gas bubbles form and appear to move slowly through the viscous liquid (between 5-10 minutes or so), remove the test tube from the water and place it in a test tube rack (careful!! The test tube may be warm!) f. While cooling, swirl the end of a wooden stick in the warm polymer mixture. Pull out the wooden stick. What do you observe? Make observations, and make a final observation of Lucite before you finish the lab after it has cooled. g. Dispose of the test tube with the Lucite on the cart after complete cooling and your final observation. II. Preparation of Nylon 6,6 – DO NOT TOUCH! DO NOT TOUCH! DO NOT TOUCH! a. Mix 2.0 mL of 20% (w/v) NaOH solution with 10 mL of 4% (v/v) 1,6-hexanediamine solution in a beaker (one of your smaller ones) b. Use a disposable Pasteur pipet (the glass ones) to slowly add about 10 mL of 3% (v/v) adipoyl chloride which is dissolved in cyclohexane to the top of the solution in the beaker. Do not violently squirt the solution in there. Attempt to lay it on top of the present solution as gently as possible. c. A film will form at the interface of these two liquids (if you do it gently!) Slowly lift and pull the film from the center with a wooden stick or a copper hook but NOT your little fingers! 7 d. Wind the film around a wooden stick and continue to pull the nylon out of solution until you have a good bit (perhaps about 1 inch in thickness if it would be balled up). Do not pull and walk – interfering with other lab groups! e. Observe the thickness of your nylon rope f. Cut the rope and immerse in a beaker of fresh water. g. What happens to the thickness of the rope? h. Remove it from the water and dry it between two paper towels. Record your observations i. Vigorously mix the contents of your beaker used to make the nylon rope – again no smelling the gas! j. Record your observations. k. Pour this mixture from the beaker into a cold water bath and wash it. Dry this nylon between two paper towels as before and record your observations. l. Place all the nylon in the used polymer solids container. Any remaining organic liquid should be poured into the used organic solutions container. All water can be poured down the drain, all paper towels can be disposed of in the trash. III. A Silly Polymer: Put on your some GLOVES!!! a. Pour 20 mL of the Elmer’s glue solution into a styrofoam cup b. Add food coloring of your choice to the glue solution (OPTIONAL) c. Add 10 mL of the crosslinker (borax solution to each cup) d. IMMEDIATELY begin stirring the solutions together using a wooden stick e. After a few minutes of mixing, the silly putty can be removed from the cup and kneaded with your hands (in gloves!!) Continue to knead until the desired consistency is reached. f. Drop the ball from a height of 30 centimeters. Measure and record its rebound height g. Stretch the silly putty slowly h. Stretch the silly putty quickly IV. I’m Melting: Polymer solubility a. Place some styrofoam pieces (already destroyed styrofoam pieces, please do not demolish our last remaining cups . . . if anyone wants to bring in some styrofoam cups that would be nice!!) inside a 400 mL beaker b. Add between 20 and 30 mL of acetone (use the larger volume if you actually use a whole cup) c. Use your glass stirring rod and stir d. What happens? Record your observations e. Remove the mass from the beaker and blot dry with paper towels. Record observations of dry material f. Dispose of your residual acetone by rinsing down the sink with extra water g. What desirable properties does this material have? 8 Results and Discussion: Turn in Results pages only!! Keep the rest of the lab, why?? because you like it soooo much Name:________________________________________ Partner: _________________________________ Polymethyl Methacrylate (Lucite, PMMA) Observations: Nylon 6, 6 Observations: Thickness of rope before water: Thickness of rope after water: Dry nylon rope: Beaker nylon observations: Slime Observations: 9 Silly Putty Observations: Rebound Height from 30 centimeters (room temperature):_________________________ Stretching observations (slow): Stretching observations (fast): Comparison: I’m Melting Observations Styrofoam before mixing with acetone: Styrofoam after mixing with acetone: Styrofoam after some drying: Desirable properties (can you see a use for this??) – HINT, this is used for something!: Conclusion: 10 Hope you had fun! 11