doc
advertisement

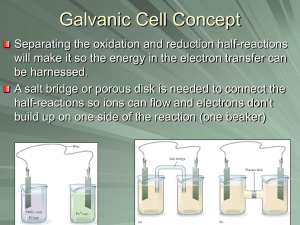
1 Electrochemistry Electrochemical cells I. Redox reactions A. involve transfer of electron from one reactant to another reactant to form products 1. reactant which loses e- is reducing agent (causes reduction) 2. reactant which gains e- is oxidizing agent (causes oxidation) B. redox reaction can be split into two separate half-reactions C. redox reaction compressed of reduction (gained electrons) and oxidation (loss of electrons) 1. Mnemonic: OIL RIG 2. Example Cu2+ (aq) + Zn (s) Cu (s) + Zn2+ (aq) a. Reduction: Cu2+ (aq) + 2 e- Cu (s) b. Oxidation: Zn (s) Zn2+ (aq) + 2eQuestion: Of the reactants, which causes oxidation? Ans. Cu2+ (aq) is the oxidizing agent. Question: Of the reactants, which causes reduction? Ans. Zn (s) is the reducing agent. D. Note that total reaction is the sum of the reduction half-reaction and oxidation half-reaction 1. in half reactions, charge must be conserved e.g. Pb4+ (aq) + 2 e- Pb2+ (aq) 2. Half reactions are added such that # e- gained = # e- lost - This is a requirement from the law of charge conservation. E. Definition: the reduced and oxidized substances in a half-reaction are called a redox couple Examples: Cu2+/Cu and Zn2+/Zn 2 F. In electrochemical cells, half-reactions occur at electrodes 1. reduction occurs at cathode – by definition 2. oxidation occurs at anode – by definition 3. mnemonic: oxidation and anode begin with vowels - Note: e- can move spontaneously or be forced to move. II. Types of electrodes A. Metal/Metal-ion 1. Metal in contact with salt 2. Half-reaction: M+ (aq) + e- M (s), Cu2+ (aq) + 2e- Cu (s) 3. Notation: M(s) | M+ (aq), Cu (s) | Cu2+ (aq) – vertical pipe indicates an interface (surface) B. Gas electrode 1. Gas bubbled over inert metal (e.g. platinum) 2. Metal serves as electron source or sink 3. Half-reaction e.g. Hydrogen electrode 2 H+(aq) + 2e- H2(g) 4. Notation: Pt(s) | H2(g) | H+(aq) - f(H2) = 1 bar and a(H+) = 1, then the electrode is the standard hydrogen electrode C. Metal/Insoluble Salt 1. Metal coated with insoluble metal salt immersed in salt water 2. Half-reaction: MX(s) + e- M(s) + X-(aq) 3. Notation: MX(s) | M(s) | X-(aq) 3 III. Cells and Potentials A. Daniell cell 1. Electrochemical cell that has a. two different redox couples b. redox couples separated by semi permeable membrane (porous pot) - negative counterlons and H+ diffuse, not metal ions porous pot Zn Cu ZnSO 4 (aq) CuSO 4 (aq) Daniell cell 2. Note electrolyte solutions (ZnSO4 (aq) and CuSO4 (aq) have an interface. B. Electrolytic concentration cell 1. Electrochemical cell that has a. same redox couple at each electrode b. different concentrations of salt at each electrode c. redox couples separated by semi permeable membrane of salt bridge 2. Also has liquid – liquid interface C. Liquid Junction Potentials 1. At liquid – liquid interface, diffusion of counterion is necessary for charge balance 2. Mobility of counterion through membrane may be slow or hindered 3. Temporary charge imbalance erects potential across membrane 4. Junction potentials becomes significant with values of 10 mV or more 5. Reduces measured potential of cell 6. An adequate solution to junction potential problem is salt bridge a. tube filled with ionic jelly b. mobility of ions going to jelly and coming out of jelly nearly equal c. nearly eliminates liquid junction potential (1 to 2 m V) 4 D. Galvanic cell 1. Electrochemical cell that contains spontaneous redox reaction 2. Potential across electrodes of galvanic cell is positive 3. Example: Cu2+ (aq) + Zn (s) Cu (s) + Zn2+ (aq) a. copper wants the e- more than zinc b. thus copper pulls away the electrons causing current 4. e- are left on electrode as metal (Zn) is oxidized, thus anode is negative and cathode is positive 5. Notation: Zn(s) | ZnSO4 (aq) || CuSO4 (aq) | Cu (s) or Zn(s) | ZnSO4 (aq) CuSO4 (aq) | Cu (s) a. read notation from left to right i. Note Zn (s) Zn2+ (aq) ii. Note (with salt bridge) (liquid junction) Cu2+ (aq) Cu (s) b. double pipe defines salt bridge c. vertical ellipses defines liquid junction 6. Value of cell potential relates work done by electrons a. we = -eE work done by e- to travel from anode to cathode i. e – charge of eii. E – cell potential (voltage) b. Recall rG = we i. Important aside: We see why electrochemistry is so important because measuring electrical work done during reaction gives us the change in the Gibbs free energy. ii. Considering the differential change in the electrical work of the system yields a) dw e r G d b) dw e eN A E d - where n = number of electrons participating in balanced redox equation - define F = eNA Faraday’s constant = 96,485 C/mol iii. Set equation a) and equation b) equal to each other to yield the relationship between cell potential and the Gibbs free energy of reaction. r G FE E. Nernst Equation 5 1. We have not considered effect of concentration so far on how voltage charges during redox reaction 2. Concentration dependence of voltage on concentration is expressed in the Nernst equation G = G + RT ln Q - F E = - F E + RT ln Q E E0 RT ln Q F At 25 C, E E 0 0.0257 V ln Q 3. standard state 1 bar, all activities are 1 4. thus Nernst equation yields voltage of cell when activities are not 1 Example: For the reaction, Cr (s) + Ni2+ (aq) Cr3+ (aq) + Ni (s), calculate the EMF when E = 0.51 V and a) [Ni2+] = [Cr3+] = 1 M First balance equation (remember conservation of charge) 2 Cr (s) + 3 Ni2+ (aq) 2 Cr3+ (aq) + 3 Ni (s) 2 Cr 3 1 Q 3 1 3 1 Ni 2 2 E E0 RT 0.0257 V ln Q 0.51 V ln 1 0.51 V 0 V 0.51 V F 6 b) [Ni2+] = 2.0 M; [Cr3+] = 0.010 M Cr Q Ni 3 2 E E0 2 3 0.010 2 1.25 105 3 2.0 RT 0.0257 V ln Q 0.51 V ln 1.25 105 0.51 V 0.048 V 0.56 V F 6 Decreasing concentration of product and increasing concentration of reactant increases the cell potential. 6. electrochemical cells at equilibrium 6 a. at equilibrium E 0 b. Nernst equation becomes E E0 RT RT ln Q E 0 ln K F F c. measuring potential order standard conditions yields equilibrium constant FE RT E ln K K e RT F 0 0 IV. Standard potentials A. We wish tabulate potential of half-reactions B. Experimentally, potentially of half-reaction can not be measured in isolation of other half-reactions C. Just as we did for Gf for ions, we need a reference D. Standard hydrogen electrode (SHE) 2 H+ (aq, 1M) + 2 e- H2 (g, 1bar) at 25 C E = 0.00 V by definition. E. All other half-reactions measured relative to SHE F. To find standard potential of cell - Cell potential is due to both oxidation and reduction. Ecell = Ered + Eox - When the half-reaction is reversed, the potential has the opposite sign. - Thus Eox has the opposite sign of the standard reduction potential. *Source of Confusion* Electrical potentials are intensive quantities. - They do not depend on stoichiometric coeff. - To find potential of cell, add potentials of half-reactions. **Source of confusion** When calculating standard cell potentials, one does not multiply standard reduction potential by stoichiometric coefficients. This method is different than the procedure to calculate Hrxn, Srxn and Grxn. 1. need half-reactions occurring at each electrode 7 Cd(s) | Cd2+ (aq) || Ni2+ (aq) | Ni (s) 2. identify where reduction and where oxidation are occurring Anode – cadmium Cathode – nickel 3. find standard potential from table Ni2+ (aq) + 2 e- Ni (s) Cd2+ (aq) + 2 e- Cd (s) E = - 0.28 V E = - 0.40 V 4. flip sign of oxidation Cd (s) Cd2+ (aq) + 2 e5. add together E = + 0.40 V Ni2+ (aq) + 2 e- Ni (s) Cd (s) Cd2+ (aq) + 2 eTotal cell potential E = - 0.28 V E = + 0.40 V E = + 0.12 V G. Note if Ecell > 0 is positive, then reaction is spontaneous ELECTROLYSIS 8 The process of adding electrical energy to make a nonspontaneous process, spontaneous. Applications of electrochemical cells Galvanic cells - source of electrical energy (battery) Electrolytic cells - refining of ores - produce alkali, alkaline earth metals - produce H2 and O2 from water (fuel cells) - metal plating (gold, chromium, etc…) Consider the formation of KBr Br2(l) + 2 e- 2 Br-(aq) K(s) K+(aq) + e- E = 1.07 V E = 2.92 V E = 3.99 V Balanced reaction is 2 K (s) + Br2 (l) 2 KBr (aq) Calculate K (equilibrium constant) E0 3.992 RT ln K K e 0.0257 e 311 10135 nF This reaction is definitely spontaneous!! Is there anyway to make K(s) and Br2(l) from KBr(s)? Yes!, by reversing the voltage, i.e., forcing e- on K+ and ripping e- from Br-. When an external voltage is applied that is greater than 3.99 V, the reverse reaction occurs. Schematic of an Electrolytic Cell EMF source (battery) flow of e- 9 K+ (l) K (l) 2 Br- (l) Br2 (g) Liquid potassium is depositing on the cathode. Gaseous bromine is forming on the anode. Note: Cell reaction is 2 KBr (l) 2 K (l) + Br2 (g). We must use the molten salt rather than the aqueous solution because if we attempt the reaction 2 KBr (aq) 2 K (l) + Br2 (g) other reactions with the solvent (water) occur. *Source of Confusion* The following table gives the signs of the electric potential at each of the electrodes in each type of cell. Cathode + - Galvanic Electrolytic Anode + However current always flows from anode to cathode. The amount of K(s) or Br2 (l) can be controlled by controlling the number of moles of electrons in an oxidation/reduction process. - The number of moles of electrons used (i.e., total charge used) is controlled by adjusting the time that the electrical current is allowed to flow. - current amount of ch arg e I time - SI unit of current is Ampere (A) - 1A 1C 1s -Q=It Q – charge I – current t – time Time(s)/Current(A) Q = It F Faraday’s Mass(g) M Molar mass Molar ratio 10 Example: An iron spoon is placed in an electrochemical cell with AuCl3 solution to be gold plated. How long must the spoon be in the electrolytic cell if the spoon is to be plated with 0.293 g of Au and the current of the cell is 1.03 A. 1st Question: How many moles of e- are needed for the reaction Au3+(aq) + 3 e- Au(s) 0.293 g 1 mol Au 3 mol e 0.00446 mol e 196.97 g 1 mol Au 2nd Question: How many coulombs is 0.00446 mole-? 0.00446 mole- 96,485 C/mol = 431 C 3rd Question: If current is 1.03 A, how long does it take for 431 C to flow? Q It t Q 431 C 418 s I 103 . C/s Example: How many grams of barium metal can be produced by the passage of 173 C of charge in an electrolytic cell of molten BaCl2? Ba2+ (l) + 2 e- Ba (s) 173 C 1 mol e 1 mol Ba 137.33 g 0123 . g Ba 96,485 C 2 mol e mol Ba