Sterile Prep Policy
advertisement

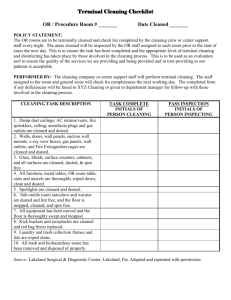
Quality Assurance for Sterile Products Pharmacy Department Policy POLICY: The pharmacy will assess and maintain the quality of products, aseptic technique, and the environment of the clean room area to comply with Chapter <797>. ASEPTIC TECHNIQUE: 1. Before being allowed to compound patient medications, each IV technician/pharmacist must successfully complete and pass the aseptic technique for IV preparation competency as well as perform a media fill test. The competency exam and media fill test must be performed annually. 2. If one fails the written exam, it must be re-taken. 3. If media growth is found during the media fill test, the proper gowning procedure and aseptic technique will be reviewed. The media fill test must be repeated. HAND WASHING: 1. Proper hand washing must be done prior to compounding of sterile products and every 4 hours thereafter during periods of active compounding. 2. Wash hands and arms to the elbows for at last 30 seconds with “a liberal amount” of antimicrobial skin cleanser. Hands should be then rinsed thoroughly. Hand washing is required upon entering the clean room. GARBING: 1. Appropriate garb is required for anyone entering the clean room. This includes pharmacists, technicians and any other housekeeping or maintenance personnel. 2. Before entering the clean room, compounding personnel must remove and outer garments that may shed; cosmetics that may shed flakes and particles; all hand, wrist or other body jewelry that could interfere with PPE (personal protective equipment). 3. The wearing of artificial nails or extenders is prohibited while working in the sterile compounding environment. 4. Personnel must don the following PPE and perform hand hygiene in an order that proceeds from dirtiest to cleanest activities. a. Shoe covers b. Head and/or facial hair covers c. Mask d. Wash Hands e. Gown f. Sterile Gloves 5. Sterile gloves shall be the last item donned before compounding begins. 6. When compounding personnel must temporarily exit the ISO 7 (cleanroom) environment during a work shift, the exterior gown, may be removed and retained in the ISO 8 anteroom/area, to be re-donned during that same work shift only. However, shoe covers, hair and facial hair covers, face masks, and gloves must be replaced with new ones before re-entering the ISO 7 clean environment along with performing proper hand hygiene. 7. Coats, purses and other personal items should be stored in employee lockers; personal items are not to be stored in the anteroom or cleanroom area. SURFACE CLEANING: 1. Shift Cleaning a. Active work surfaces and counter tops are cleaned with 70% IPA (isopropyl alcohol) during each shift (includes surfaces of hoods, countertops, and supply carts). b. Hoods are cleaned with 70% IPA at the beginning of each shift and whenever necessary. c. Spills and residue in the hood and adjacent surfaces should be disinfected with water and 70% IPA at least every 30 minutes during active compounding. d. Supplies placed in the hood environment must be unpackaged in the anteroom area and wiped with a sanitizing agent, such as sterile 70% IPA. e. Routine application of 70% IPA should occur throughout the compounding day and whenever non-sterile surfaces (vials, counter tops, chairs, and carts) are touched. 2. Daily Cleaning a. Floors in the buffer area, as well as the anteroom area, should be mopped once daily, when no aseptic operations are in progress. Mopping should be performed by trained custodial personnel using approved agents described in written procedures. b. All cleaning tools, such as wipers, sponges, and mops are nonshedding and dedicated to use in the buffer or clean area. Floor mops may be used in both the buffer or clean area and anteroom area, but only in that order. Most wipers are discarded after one use. If cleaning tools are reused, their cleanliness is maintained by thorough rinsing and sanitization after use and by storing in a clean environment between uses. c. Trash receptacles should be emptied daily or more frequently according to the needs of the area. d. The vertical flow hood (used for chemo/hazardous drugs) is cleaned daily with a bleach first to deactivate hazardous materials, and then with 70% IPA to disinfect the hood. 3. Weekly Cleaning a. Exterior surfaces of the laminar-airflow hood should be cleaned weekly with 70% isopropyl alcohol b. Bins/trays used to transfer medications in and out of sterile compounding areas must be cleaned at least weekly with bleach first, and then 70% IPA. i. Chemo transport box ii. Chemo storage bin located on patient floors 4. Monthly Cleaning a. Storage shelving in the anteroom should be emptied of all supplies, and then cleaned and sanitized using 70% isopropyl alcohol. b. Prefilters in the laminar-airflow hoods should be cleaned monthly. AIR & SURFACE SAMPLING 1. Air Sampling: performed every 6 months 2. Surface Sampling: performed every month 3. Any positive sample contamination will be sent to a lab to be identified 4. Growths greater than the predefined action points require investigation into the source of contamination a. Staff will be informed and the a defined remediation plan will be implemented (see form attached) b. Possible sources to investigate: ventilation and heating systems, HEPA filters, handwashing, and garbing procedures c. The source of contamination will be eliminated and the area will be resampled d. Highly pathogenic microorganisms require immediate remedy (gramnegative rods, coagulase-positive staphylococcus, molds, and yeasts) i. Housekeeping will be contacted to do a thorough cleaning of the contaminated area ii. Infection Control may be contacted to aid in management and rotation of disinfection agents