SUMMARY OF PRODUCT CHARACTERISTICS
advertisement

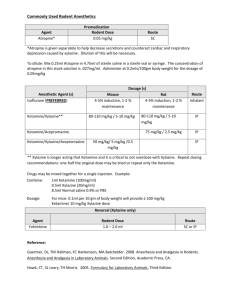
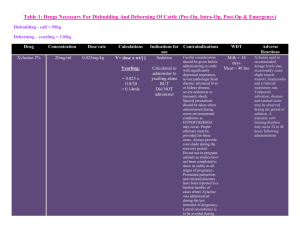
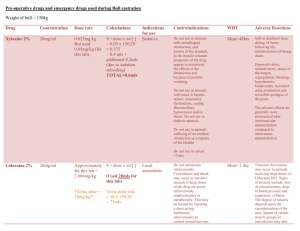
SUMMARY OF PRODUCT CHARACTERISTICS 1. Product: Xylazine 2% 2. Qualitative and Quantitative Composition: Xylazine Base 20 mg/ml Antimicrobial Preservatives: Methyl parahydroxybenzoate Propyl parahydroxybenzoate 1.8 mg/ml 0.2 mg/ml 3. Pharmaceutical Form: Solution for injection 4. Pharmacological Properties: Xylazine 2% is an injectable solution containing Xylazine Base 20 mg/ml. Xylazine is an alpha/2- adrenergic drug with sedative, analgesic and muscle relaxing properties which acts via the CNS. Xylazine is thought to act by activation of the central presynaptic alpha/2- receptors. Activation of these central alpha/2- receptors seems to regulate central dopamine and nerepinephrine storage or release. Xylazine’s analgesic and sedative actions are related to its central nervous system depression, while the muscle relaxant effects are due to the inhibition of the intraneural transmission of impulses in the central nervous system. Onset and duration of sedation is species-dependent, varying from 5-15 minutes in horses, to 15-30 minutes in dogs and cats. Duration of sedation is from 40 minutes to 2 hours. ATC vet code: QN05CM93. 5. Clinical Particulars: 5.1 Target Species: Cattle, horses, dogs and cats. 5.2 Indications for use: Xylazine 2% is a sedative with analgesic and muscle relaxant proprieties for use in cattle, horses, dogs and cats where sedation is required including: 1. 2. 3. 4. Handling fractious animals e.g. for transportation. Medical examinations e.g. x-ray examinations, removal of bandages; examination of the penis and oral cavity. Premedication for minor superficial operations, and local or regional anaesthesia. Elimination of defecation when examining and treating the vagina, uterus and hindquarters. Summary of Product Characteristics Page 1 of 5 5.3 Contra-indications: Not to be used in horses intended for human consumption. Not for use in animals from which milk is produced for human consumption. Do not administer by the intra-carotid route. Careful consideration should also be given before administering to animals exposed to stress conditions such as extreme heat, cold high altitude or fatigue. 5.4 Undesirable Effects: Following the use of Xylazine 2% in cattle profuse salivation, bloat and polyuria may occur. Side effects such as bradycardia, cardiac arrhythmia and polyuria may occur in the horse. Following intravenous administration to horses, a transient rise followed by a fall in blood pressure usually occurs. Vomiting is commonly observed in dogs and cats following use of the product. Tympany should be avoided in recumbent cattle by maintaining the animal in sternal recumbency. Provision should also be made for facilitating dependent drainage from the mouth to avoid inhalation asphyxia. 5.5 Special precautions for use: Care should be exercised in administration to aged or debilitated animals. 5.6 Use during pregnancy and lactation: Xylazine 2% should not be administered during the later stages of pregnancy because of the risk of inducing premature parturition. As the safety of xylazine use during organogenesis has not been fully demonstrated by current methods, it should be used with caution during the first month of pregnancy. 5.7 Interaction with other medications and other forms of medication: None known. 5.8 Posology and method of administration: Cattle: Xylazine 2% is given by intramuscular injection. The dose rate is 0.05-0.3 mg/kg (0.25-1.5 ml/ 100 kg) bodyweight, according to the degree of sedation required. Very Summary of Product Characteristics Page 2 of 5 fractious animals may require the higher dose rates not exceeding 0.3 mg/kg (Dose rate 4). Dose 1 2 3 4 mg/kg 0.05 0.10 0.20 0.30 mg/50 kg 2.5 5.0 10 15 ml/50 kg 0.12 0.25 0.50 0.75 Horses: Xylazine 2% is given by slow intravenous injection. Dosage depends upon the degree of sedation required and the response of the animal and is 0.6-1 mg/kg (3-5 ml/100 kg) bodyweight. Nervous or excitable horses may require higher doses. Older horses and those having undergone severe physical exertion before treatment should receive the lowest dose rate. The horse does not usually become recumbent with Xylazine 2% and light to deep sedation with variable degree of analgesia is obtained. Effects are usually seen within 5 minutes and persist for approximately 20 minutes. Xylazine 2% may be employed in the horse as a premedication to barbiturate anaesthesia or in combination with regional or local anaesthesia. Dogs: Xylazine 2% is administered intramuscularly at dose rates of 13 mg/kg (0.05-0.15 ml/kg bodyweight). It may be used in combination with a local anaesthetic. Premedication with atropine may be desirable in some cases. Xylazine 2% is synergistic with the barbiturates and reduces the dosage of the latter by approximately one half. Cats: Xylazine 2% is administered intramuscularly at a dose rate of 3 mg/kg (0.15 ml/kg bodyweight). Premedication with atropine may occasionally be desirable. 5.9 Overdose: Alpha-2 blockers such as atipamezole are effective in reversing the sedation and other physiological effects of xylazine. 5.10 Special warnings for each target species Cattle are about ten-times more sensitive to Xylazine than horses and high doses will cause recumbency. Special precautions to minimize regurgitation, inhalation pneumonia and bloat should be taken during anaesthesia. Summary of Product Characteristics Page 3 of 5 5.11 Withdrawal periods: Cattle: Meat – 14 days Not for use in cattle producing milk for human consumption. In accordance with the Horse Passport legislation (Commission Decision 2000/68/EC as implemented in national legislation) a 6 month withdrawal period applies to the use of this product 5.12 Special precautions to be taken by the Person Xylazine is an alpha adrenoceptor agonist acting primarily on alpha-2 receptors. Care should be taken to avoid accidental injection/ self injection. Wash splashes from skin and eyes immediately. In the event of accidental injection/ self injection, seek urgent medical advice and show the label and other product literature. DO NOT DRIVE as sedation and changes in blood pressure may occur. It is recommended that, once the required dose has been withdrawn from the vial, the needle should be kept guarded until the product is administered. Alternatively, it should be removed from the syringe and immediately inserted into the injection site and the syringe should then be connected to it. Advice to doctors Xylazine is an alpha adrenoceptor agonist whose toxicity may cause clinical effects including sedation, respiratory depression and coma, bradycardia and hypotension and hyperglycaemia. Verticular arrhythmias have also been reported. Treatment should be supportive with appropriate intensive therapy. 6 Pharmaceutical Particulars 6.1 Incompatibilities None known 6.2 Shelf-life 5 years 6.3 Special Storage Precautions Do not store above 25o C. Following withdrawal of the first dose, use the product within 28 days. Discard unused material. Summary of Product Characteristics Page 4 of 5 6.4 6.5 7.0 . Nature and contents of container Amber vials, Type 1 Ph. Eur glass, containing 25ml. Elastomeric closures 20 mm. Aluminium seals with removable centres. Special precautions for disposal of unused product and waste material: Any unused product or waste material should be disposed of in accordance with national regulations. Additional Information: 7.1 Name and address of Marketing Authorisation Holder: Millpledge Pharmaceuticals Whinleys Estate Church Lane Clarborough Retford Notts DN22 9NA Legal Category Date of revision of SPC Marketing Authorisation No.: Date of renewal: Summary of Product Characteristics POM 10/10/2005 Vm 04409/4006 12/10/2004 Page 5 of 5