math_component03_26 - University of South Alabama

Computational Model of Tumorigenesis
We use a cell-based, spatially explicit computational model to test and simulate the predictions of the hypothesized two-step mechanism for tumor progression. A spatially explicit model is necessary to describe the special cell geometry of cells in tissue recombination allografting experiments, in which epithelial cells line the ducts and stromal cells are scattered at a lower density in the region between ducts. Although the experimental system is 3-dimensional, as a first approximation we model a 2-dimensional cross-section of the allograft. Initial cell positions were taken directly from the positions of cell nuclei in a 2-dimensional experimental image of an allografted prostate duct in which cells were labeled as epithelial or stromal (Figure 3). In a cell-based model, we assume that cells produce morphogens and morphogens diffuse exclusively in the region between ducts.
The computational model defines five cell types (normal and altered stromal cells; normal, proliferative and invasive epithelial cells) and simulates the transformation of epithelial cells in response to morphogens secreted by stromal cells. Initially all epithelial cells are normal and stromal cells are randomly identified as normal or altered for different fractions of altered stromal cells, as per the experiment. We do not include cell division, apoptosis, or cell movement. At each time step the following steps occur: i.
Stromal cells produce morphogens: normal stroma produce M2 and altered stroma produce M1 in a basic model; both cell types may produce both morphogens in a more sophisticated implementation. ii.
Morphogens diffuse throughout the morphogenetic domain. iii.
At threshold morphogen levels, epithelial cells transform. Epithelial cells transform from normal to proliferative at threshold levels of M2, and transform from proliferative to invasive at threshold levels of M1.
Computational Model Results
This section is intended to be a description only of the computational model results in general/ for all parameters. Then in the next section we will describe how we choose experimentally relevant parameters .
A simple implementation of the two-step mechanism in a computational model yielded results that were qualitatively similar to experimental observations. In computer simulations, the ratio of stromal cells labeled ‘altered’ or ‘normal’ was varied from 0 to 1, analogous to varying the fraction of altered stromal cells in experiments. The measured response variable for all simulations was the fraction of epithelial cells that transformed
(step 1 proliferative or step 2 invasive). The fraction of transformed epithelial cells results for a typical set of simulation parameters is shown in Fig 4A. For 100% normal stromal cells, there were no associated transformed or invasive epithelial cells. For 100% altered stromal cells, there were only transformed epithelia (PIN). As in the experimental scnerario, the computational model yielded the greatest invasion at heterogeneous mixtures of stromal cells.
Running a simulation required choosing the values of 8 parameters: the production rate, diffusion rate and decay rate of each morphogen, and the threshold levels at which epithelial cells responded to morphogens. Different choices of parameters resulted in different steady state morphogen levels and varied the dispersive range of each morphogen (see Methods for details). Initially in simulations, all morphogen levels were zero. Morphogen levels then increased until they approached steady state levels; however, there were fluctuations due to random walk on the lattice and spontaneous decay. Depending upon the choice of parameters for morphogen 1, the levels of M1 at the location of healthy epithelial cells may have been high enough to transform a number of epithelial cells from normal to proliferative. Likewise, depending upon the choice of parameters for morphogen 2, the levels of M2 at the location of proliferative epithelial cells, if any, may have been high enough to transform these epithelial cells from proliferative to invasive. Due to the two-step mechanism, the number of epithelial cells that become proliferative (Step 1 transformation) are independent of all morphogen M2 parameters and the number of epithelial cells that become invasive (Step 2 transformation) were always less than or equal to the number that become proliferative.
It is noteworthy that for many parameter values with low morphogen signal strengths, no epithelial cells were transformed. A global sensitivity analysis, varying all parameters systematically and also randomly, found that all parameters yield qualitatively similar results in which the number of proliferative cells, if non-zero, increased monotonically with the fraction of altered stroma and the number of invasive cells, if non-zero, was biphasic with a maximum at intermediate fractions of altered stroma, as in Fig 4A.
Wnt and SDF1 are potential candidates for stromal signals
In vitro experiments were performed to test the biologic viability of the modeling results. Previous reports suggested Wnt3a and SDF-1 as candidate morphogens to supplant M1 and M2 designations, respectively. Both Wnt3a and SDF-1 were produced dominantly by stromal cells and has been known to support transformation and cancer progression. Wnt3a was expressed approximately 4-fold greater in Tgfbr2 fspKO
prostatic stromal cells compared to Tgfbr2 floxE2/floxE2
cells by real-time RTPCR. However, cultured
Tgfbr2 floxE2/floxE2 stromal cells had 5-fold greater SDF-1 expression by realtime RTPCR.
Two experiments were designed to determine the impact on Wnt3a and SDF-1 expression when Tgfbr2 floxE2/floxE2 and Tgfbr2 fspKO
prostate stromal cells were cultured together. In mixtures of Tgfbr2 floxE2/floxE2
and Tgfbr2 fspKO stromal cells, Wnt3a mRNA levels increased in a linear manner with increasing percentage of Tgfbr2 fspKO prostate stromal cells co-cultured at ratios of 0%:100%, 25%:75%, 50%:50%, 75%:25%, and 100%:0. In co-cultures of Tgfbr2 floxE2/floxE2
and Tgfbr2 fspKO stromal cells, SDF-1 mRNA levels decreased in a linear manner with increasing percentage of Tgfbr2 fspKO prostate stromal cells in the mixture. Together this data suggested that Tgfbr2 fspKO
stroma were dominant producers of Wnt3a and the Tgfbr2 floxE2/floxE2 cells were the dominant producers of SDF-
1. To quantitate the cellular production of the two factors by the individual cell types the stromal cells were FACS sorted based on CMFDA (green) dye labeling of the Tgfbr2 fspKO cells. Following co-culture incubation at the previously described ratios RT-PCR was performed for Wnt3a and SDF-1 in the Tgfbr2 floxE2/floxE2
and Tgfbr2 fspKO
stroma individually by real-time RTPCR. Wnt3a results…. Figure 5A
As before, SDF-1 mRNA levels for Tgfbr2 floxE2/floxE2 prostate stromal cells were
5-fold greater than Tgfbr2 fspKO
prostate stromal cells when grown alone in culture.
However, FACS sorting of Tgfbr2 floxE2/floxE2
stromal cells grown in 75%:25% and
50%:50% co-culture with Tgfbr2 fspKO
revealed approximately 8-fold increase in SDF-1 mRNA expression compared to Tgfbr2 fspKO mRNA levels of the Tgfbr2 fspKO cells grown alone. Interestingly, SDF-1 stromal cells were elevated 2-fold as a result of coculture (75%:25%, 50%:50%, and 25%:75% ratios) over Tgfbr2 fspKO
cells grown alone.
The results suggested that both Tgfbr2 floxE2/floxE2 and Tgfbr2 fspKO stromal cells may cooperatively production of one or more autocrine factors resulting in increased SDF-1 mRNA levels. Further support for the presence of a soluble mediator of stromal cooperation was determined by culturing the Tgfbr2 floxE2/floxE2
and Tgfbr2 fspKO
stromal cells on the upper and lower chambers of a Boyden chamber-like set-up separating the two cell types grown in common media conditions. (more details here) Figure 5B
Our computational model was modified to reflect the measured levels of Wnt3a and SDF-1 as putative morphogens M1 and M2. In the original two-step model considered above, M1 production by normal stromal cells and M2 production by altered stromal cells was 0 while in the modified model, each stromal cell type produced a second morphogen at levels relative to that of the other cell type as prescribed by the data in Fig 5A and 5B. In order to replicate experimental observations when both stromal cell types produced both morphogens, it was important to require that the threshold levels for transformation were sufficiently high so that low levels of M1 produced by normal cells
would not cause epithelial cells to become proliferative and low levels of M2 produced by altered stromal cells would not cause cells to become invasive. Provided this condition, results for the two models were identical. In particular, the simulation parameters used in Fig 4B incorporated into the modified model were indistinguishable within experimental error. Adjusting only the threshold levels at which epithelial cells transformed in response to M1, we found that for threshold levels below ??, the probability of invasive epithelial transforrmation for 100% normal stroma was greater than 1%. Thus, our modified model predicted that for sufficiently high thresholds of morphogen transformation that are free parameters in our model, Wnt3a and SDF1 are potential morphogens that fit the respective M1 and M2 designations given their experimentally measured levels.
%this paragraph was moved from previous section and needs to be more smoothly integrated
Fig 4C shows that the fraction of epithelial cells that become proliferative (step 1) decrease monotonically as the decay rate of M1 is increased. In order to identify a choice of parameters that yield approximate the number of cells transformed in vivo, we adjusted the decay rates of morphogens M1 and M2 so that approximately 80% of epithelial cells become proliferative and 20% become invasive at 50-50 mixed levels of stroma (Fig 4B). This set of parameters for the approximation is non-unique since different parameters could have been adjusted for the same phenomenological fitting.
Epithelial Cell Fate Depends Upon Tissue Position
Since our computational model is spatially explicit, averaging results over multiple simulations can yield predictions about which epithelia cells are most vulnerable to transformation. Given a fixed pattern of stromal cell locations, but many different random assignments for which stromal cells are normal or altered, some epithelial cells consistently transform with a relatively high probability while other epithelial cells never transform. This means that even without knowing the exact pattern of altered stroma cells, the model makes a prediction about which epithelial cells are most likely to transform. In Fig 6A, the epithelial cells that are most likely to become proliferative (Step
1) when the fraction of altered stroma is 0.5 are shown in red. The cells most likely to transform are located near high density areas of stroma. This is presumably due to the increased likelihood of both stromal cell types in this area (stromal heterogeneity). In vivo, it is likely that only one stromal cell would be altered. Fig 6B shows the relative rates of epithelial transformation when there is exactly one altered stroma cell at a random stromal location. The epithelial cells most likely to transform are shown in red while the epithelial cells that never transform are shown in gray. Again, the epithelial cells most likely to transform are located near areas of high stromal density.
Necessity of the Normal Stroma Making M2 and whether or not the stroma cell types act independently
In the original and modified two-step models, we have made an assumption that only the normal stroma makes significant levels of M2. However, it is possible that M2 is
made by the altered stroma and its production depends on the normal stroma. In simulations, we investigate the predictions of different assumptions for the production of
M2. Figure 7 shows the rates of transformation verses the fraction of altered stroma for cases in which (i) the altered stroma produces M2 at fixed levels independently of normal stroma, (ii) the altered stroma produces M2 at fixed levels only if the normal stroma is present, (iii) the altered stroma produces M2 at levels that increase linearly with the fraction of normal stroma. Model results are qualitatively consistent with experimental data if there is no epithelial transformation for 100% normal cells, only epithelial proliferation for 100% altered stromal cells, and both stages of transformation
(proliferation and invasion) for 50% altered stromal cells. Unsurprisingly, the first scenario contradicts the experimental data, confirming that altered stroma cannot produce
M2 independently of the normal stroma. Scenarios (ii) and (iii) are difficult to distinguish from the experimental data, suggesting that M2 may be produced by either stromal cell type, as long as the production depends the presence of the normal stroma. Also we cannot tell at this level of experimental detail if M2 production is flat or depends synergistically on the mixture of cell types.
DISCUSSION
We developed a hypothetical two-step mechanism for tumor progression based on experimental observations and used a computational model to evaluate the mechanism in silico. We find that the proposed two step mechanism can result in the observed experimental data in a simplified scenario, and that Wnt-3a and SDF-1 are viable putative key morphogens.
The computational model necessarily provides only a very simplified model of the prostate. However, as an agent-based model it is very flexible and can incorporate further levels of detail as experimental data is obtained. For example, the model could be extended to a 3D model with 3D coordinates of cell locations and could include cells with more realistic shapes by using a finer grid resolution. Stromal cells are highly elongated and this would result in an extended region of morphogen production. The orientation of elongated cells would also be expected to affect their communication.
Other morphogen interactions could be added to explore the effects of intermediate morphogen signaling.
There is an emerging paradigm of natural selection in the evolution of tumor microenvironments through cooperation. Cooperation between individuals and groups is paralleled by many levels of organized niches in biology including genomes, tissues, and animal societies and involves an exchange of resources.
I don’t believe we have this here…?
We initially described our two-step model for tumor progression using two generalized morphogens M1 and M2 that progress the system from a ‘state A’ to a ‘state B’ and then from ‘state B’ to ‘state C’. We have done this for two reasons. First, Wnt3 and SDF1 are putative morphogens for M1 and M2. It is does not alter the conclusions of our computational model if either morphogen is re-identified with another diffusing chemical. We only require that M1 and M2 are local signals, M1 production is dependent upon the altered stroma and M2 production is dependent upon the normal stroma.
Another reason we have described the tumor progression so generally is that it may apply to any other systems in which staged tumor progression depends upon more than one cell type. It is particularly important here that normal cells are required to proceed from ‘state
B’ to ‘state C’. While a homogeneous population of normal stromal cells will not result in the transformation of normal epithelia to proliferative epithelia (tumor suppressive qualities of normal cells) they are required to proceed to invasive epithelia (tumor promoting qualities).
The ubiquitous growth factor TGF-ß has often been characterized to be tumor suppressive in un-initiated epithelial cells, yet support tumor invasion as carcinogenesis progresses. Fig R shows the roles of TGF-ß hypothesized in this experimental system.
First, TGF-ß is tumor suppressive in normal stromal cells since it represses the secretion
of Wnt3a (and HGF and others). Altered stromal cells produce Wnt3a as a result of the knock out of TGF-ß responsiveness. This is ‘state B’. Fascinatingly, once the system is in
‘state B’, TGF-ß becomes cancer promoting as it stimulates wild type stromal cells to produce SDF, which drives the system to ‘state C’ towards invasion. TGF-ß also is reported to promote the expression of the cognate SDF1 receptor, CXCR4 in epithelial cells.
Our model makes the simplifying assumption that the wild type and altered stroma secrete Wnt and SDF independently. However, when more complex cases are considered
(Fig 7), we find that the experimental observations modeled in this paper can still be qualitatively reproduced. Thus independence of the stroma is not necessary in this model and, indeed, there is preliminary experimental evidence to suggest that SDF production is augmented in the mixed population (data not shown). Also, our model only follows the effect of the stroma upon the epithelium. Normal and transformed epithelial cells send signals to the stroma as well. Our model includes altered stromal cells, which are stromal cells that have altered in response to tumorigenic epithelial signals. We model thr effect of altered stromal cells on normal healthy epithelia cells. Onviously, in vivo, these altered stromal cells would be likely to have unhealthy epithelia cells nearby (perhaps already in a state analogous to state B).
It is convenient to use fixed cell positions and prescribe those positions from a “tissue stain” in order to accurately describe stromal cell positions. Using experimentally prescribed cell positions enables model simulations with realistic cell positions without a detailed understanding of how those cell positions are determined, which is beyond the scope of this project. For these same considerations, we did not model cell diffusion or proliferation. Cell diffusion and cell proliferation would both have the effect of extending the region of influence of a cell’s signal. It is possible that cell diffusion and proliferation are slow compared to the time scale of transformation so that the region of influence is mostly determined by the rate and range of morphogen diffusion.
In simulations, cells produce morphogens that diffuse, creating microenvironments that induce transformation in neighboring cells. In our simulations, the morphogen production of a single stromal cell may not be strong enough to result in epithelial transformation, yet, in that case, more than one stromal cell may signal in tandem to result in transformation. Also, diffusing cells would alter the results of the model since they would experience different micro-environments over time, and bring a memory of the previous microenvironment with them.
In our model, epithelial cells have the highest chance of becoming invasive if they are near a high density of stromal cells. This was because there would be a higher chance of having an altered stromal cell in that location. In vivo, this would be analogous to there being a higher probability of a cell randomly acquiring mutations in that location. In our mouse nearly 80% of the prostate epithelia are affected to some level going from A to B.
Going from B to C maybe around 10-25% affected. These percentages are outrageously high compared to real life by at least 2 orders of magnitude. With that said, people often
have more than one PIN lesion to suggest if they have the propensity to have one lesion, then they are likely to have others. This line of thinking really plays into the role of the stroma in all of this, since it is the stroma that connects the ducts. Indeed, the epithelial cells most likely to transform may be on more than 1 duct, but these ducts are connected to the same stromal region. Different epithelial cells experience different microenvironments not only due to their proximity to a single randomly dispersed altered stromal cells but also that of a group of stromal cells in that region. Invasion will occur at the interface of altered and healthy stroma rather than a location with 100% altered stroma.
Computational Model
In our spatially explicit computational model for two-step tumorigenesis, the production, diffusion and decay of morphogens are modeled on top of a static 2D background of stromal cells, epithelial cells and ducts. To define a biologically relevant geometry, an experimental image (Fig 3A) was used to define point positions for 209 epithelial cells,
72 stromal cells and the boundary curves of 5 ducts.
Morphogen Production, Diffusion and Decay
Stromal cells are point sources for morphogens M1 and M2.
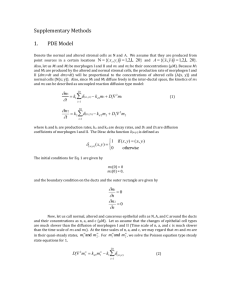
(1) equations need to by typed in..
(2)
In a simple model, N_M1 and A_M2 are zero. In a more sophisticated model, the ratios of N_M1, N_M2, A_M1, A_M2 are defined by experimental data.
The ducts are irregular in shape and define a region of diffusion with complex boundaries. Morphogens diffuse within the inter-ductal region:
(3)
Boundary conditions for the diffusive domain are reflective (e.g., like the cushions of a pool table), so that particles (cells or molecules) cannot diffuse beyond the duct boundary. Diffusion in a region with complex boundary conditions can be solved using a finite element method. We use the PDE toolbox in Matlab to solve (2) with boundary conditions (3).
Steady State Morphogen Concentrations
Epithelial cells respond to morphogens M1 by transforming from normal to proliferative, and from proliferative to invasive, at threshold loves of M1 and M2. Diffusion is a fast process and we it is reasonable to assume that the rate of diffusion is much faster than rates of cell transformation (refs). Thus, we consider the epithelial cell response to steady state morphogen levels.
While the morphogen concentration is solved as described above, we already know some things about the steady state solution. From initial zero levels of morphogen, each morphogen-producing cell adds morphogen to the diffusive area until morphogen production is balanced by morphogen decay; the steady-state total amount of morphogen contributed is c*P/d where P is the morphogen production rate, d is the morphogen decay rate and c is the number of cells producing that morphogen. Thus, total morphogen levels increase with the number of cells producing that morphogen and the production rate and decrease with the decay rate. The mean squared displacement of morphogen from its source (a mophogen-producing cell) is the product of the morphogen’s mean lifetime
(1/d) and the mean squared displacement of the morphogen per unit time D. Thus the
mean squared displacement of morphogen from its source is proportional is the mean squared displacement of the morphogen per unit time and inversely proportional to the decay rate.