Supplementary Methods file for Young, Fernàndez and Fleagle (2009)
advertisement

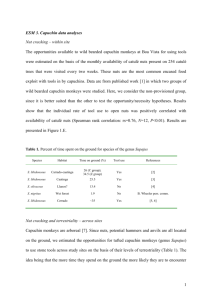
Supplementary Methods file for Young, Fernàndez and Fleagle (2009) Animal husbandry and sample composition The data for this study were taken from a mixed-longitudinal series of radiographs and associated morphometrics originating from a long-term study of capuchin monkey growth at the Harvard School of Public Health. This dataset has constituted the primary resource for research on capuchin monkey growth for more than 30 years (Ausman et al. 1982; Cole 1992; Elias and Samonds 1977; Fleagle and Samonds 1975; Fleagle et al. 1975; Fleagle and Schaffler 1982; Jungers and Fleagle 1980; Samonds and Hegsted 1973; Thurm et al. 1975; Watts 1990; Wood 2003; Young 2005). Newborn monkeys were removed from their mothers following birth and were hand-reared for 3-5 years under controlled conditions in a primate nursery. All animals were fed a diet known to promote optimal growth, housed in non-restrictive cages that were large relative to body size, and were permitted frequent (2-3 times per week) periods of exercise on a large tree positioned in one corner of the laboratory (Thurm et al. 1975; K. Samonds and C. Samonds, pers. comm.). Lateral-view uniplanar radiographs and morphometric measurements were taken weekly during the first eight weeks of life, biweekly during the following year, and at one to two month intervals for several years thereafter. Radiographic and placement procedures have been described in detail elsewhere (Fleagle and Samonds, 1975; Thurm et al., 1975). From this larger project, a subset of 15 male C. albifrons and three male C. apella were selected for the current study (i.e., all available control group males from the original study). Data from animals younger than one month of age were excluded due to difficulty identifying long bone epiphyses. In total, measurements were recorded on 493 C. albifrons radiographs and 147 C. apella radiographs. Measurements were combined across individuals to create speciesspecific mixed longitudinal samples spanning the first five years of life (Table S1). In the wild, capuchin monkeys typically wean between 9 and 14 months of age and begin reproductive activity at around three years of age (Robinson and Janson 1987). The sample used in this study therefore includes animals of all age stages, from infant to adult. Measurement protocols Right humeral and femoral maximum lengths, total (i.e., external) diameters, and medulary (i.e., internal) diameters were measured on digitally scanned radiographs (resolution: 150 ppi) using NIH ImageJ software (Abramoff et al. 2004). Brass scale bars, cut at millimeter intervals and placed in each radiograph, were used to calibrate measurments from pixels to millimeters. Radiographs were discarded in cases where it was difficult to distinguish between left and right limbs or when the proximal or distal ends of the limbs were blurred. In addition, radiographs were not used when the date the radiograph was taken and the date body weight was measured differed by more than 10 days. Humerus length was measured as the distance from the most proximal point of the humeral head and the most distal end of the trochlea, whereas femur length was measured as the to the distance from the most proximo-dorsal point on the femoral head to the most distal point of the condylar surfaces. External and “internal” (i.e., medullary) diameters were measured at midshaft on an axis perpendicular to the longitudinal axis of the bone. Measuring diameters at midshaft ensured that measurements were easily repeatable and that measurements of humerus diameter were not biased by inclusion of the deltopectoral crest. To facilitate the identification of bone-air boundaries, image edges were also enhanced using the ImageJ “Find Edges” macro prior to taking diameter measurements. Interrater reliability was high across all length and diameter measurements (Kendall’s [63]=0.78-0.93, all p<0.001). Statistical methodology To investigate the ontogenetic scaling of cross-sectional dimensions, log-transformed limb measurements were fit to the standard allometric power function: log Y = log + log X where Y represents the parameter of interest, X is an overall size variable, is the slope (i.e., scaling coefficient) and is the intercept. Allometry was recognized if the 95% confidence intervals on scaling coefficients did not encompass the expected values for isometric scaling (as defined in Table 1 of the manuscript). Age-related changes in GSF were fit to a non-linear Gompertz model (German and Meyers 1989): Y Aebe kt where Y represents the parameter of interest, t is postnatal age in days and e is the base of the natural logarithm. A, b and k are constants, calculated iteratively, that are respectively proportional to the lower asymptote of the curve, the exponential rate of decay and the tangential slope at birth. Capuchin monkeys exhibit a pronounced growth spurt at approximately three years of age, coincident with the onset of sexual maturity (Leigh 1996; Robinson and Janson 1987). Such a pronounced growth spurt necessarily affected Gompertz fits, possibly confounding growth assessments at earlier ages. Therefore, when examining age related changes in GSF we truncated the dataset to only include measurements taken prior to three years of age, at which time the monkeys had reached approximately 70% of adult body mass. We adopted a mixed-effects approach when fitting all regression models (Pinheiro and Bates 2000), allowing us to formally incorporate “random” intraspecific variation and accommodate autoregressive and heteroscedastic error structures. For each relationship of interest, a variety of models, correlation structures and error structures were tested using iterative maximum likelihood estimation, retaining the model with highest explanatory power, as gauged by comparing Akaike Information Criteria across models. The goodness-of-fit for each was also assessed using a likelihood-ratio reformulation of the standard coefficient of determination (i.e., R2): 2 R 2 (log LUM log LRM ) N where logLUM is the log-likelihood of the full model and logLRM is the log-likelihood of the intercept-only model (Magee 1990). All statistical procedures were implemented using the freely-available, open source R Statistical Package (R Development Core Team 2008). Table S1. Composition of the longitudinal radiographic samples in Cebus albifrons and C. apella Cebus albifrons (N=15) Cebus apella (N=3) Minimum Maximum Median Minimum Maximum Median Starting age (days) 30 344 41 34 40 39 Duration (years) 1.3 5.5 3 4.3 4.7 4.5 N radiographs 15 52 35 48 50 49 References Abramoff MD, Magelhaes PJ, and Ram SJ. 2004. Image processing with ImageJ. Biophotonics Int 11:36-42. Ausman LM, Powell EM, Mercado DL, Samonds KE, Lozy M, and Gallina DL. 1982. Growth and developmental body composition of the cebus monkey (Cebus albifrons). Am J Primatol 3:211-227. Cole TM. 1992. Postnatal heterochrony of the masticatory apparatus in Cebus albifrons and Cebus albifrons. J Hum Evol 23:253-252. Elias MF, and Samonds KW. 1977. Protein and calorie malnutrition in infant cebus monkeys: growth and behavioral development during deprivation and rehabilitation. The American Journal of Clinical Nutrition 30:355-366. Fleagle JG, and Samonds K. 1975. Physical growth of cebus monkeys (Cebus albifrons) during the first year of life. Growth 39:35-52. Fleagle JG, Samonds KW, and Hegsted DM. 1975. Physical growth of cebus monkeys, Cebus albifrons, during protein or calorie deficiency. The American Journal of Clinical Nutrition 28:246-253. Fleagle JG, and Schaffler MB. 1982. Development and eruption of the mandibular cheek teeth in Cebus albifrons. Folia Primatol (Basel) 38:158-169. German RZ, and Meyers LL. 1989. The role of time and size in ontogenetic allometry: I. Review. Growth Dev Aging 53:101-106. Jungers WL, and Fleagle JG. 1980. Postnatal growth allometry of the extremities in Cebus albifrons and Cebus apella: a longitudinal and comparative study. Am J Phys Anthropol 53:471-478. Leigh SR. 1996. Evolution of human growth spurts. Am J Phys Anthropol 101:455-474. Magee L. 1990. R2 measures based on Wald and likelihood ratio joint significance tests. Am Stat 44:250-253. Pinheiro JC, and Bates DM. 2000. Mixed-Effects Models in S and S-PLUS. New York, NY: Springer. R Development Core Team. 2008. R: A language and environment for statistical computing. 2.7.1 ed. Vienna, Austria: R Foundation for Statistical Computing. Robinson JG, and Janson CH. 1987. Capuchins, squirrel monkeys, and atelines: socioecological convergence with old world primates. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, and Struhsaker TT, editors. Primate Societies. Chicago and London: The University of Chicago Press. Samonds KW, and Hegsted DM. 1973. Protein requirements of young cebus monkeys (Cebus albifrons and apella). American Journal of Clinical Nutrition 26:30-40. Thurm D, Samonds KW, and Fleagle JG. 1975. An atlas of the skeletal maturation of the cebus monkey: the first year. Boston: Harvard School of Public Health. Watts ES. 1990. A comparative study of neonatal skeletal development in Cebus and other primates. Folia Primatol (Basel) 54:217-224. Wood HT. 2003. Energetics, encephalisation and Weaning: Modelling Growth and Maturation in Primate and Human Evolution [Ph.D. Dissertation]. University College London: University College London. Young JW. 2005. Ontogeny of muscle mechanical advantage in capuchin monkeys (Cebus albifrons and Cebus apella). J Zool Lond 267:351-362.