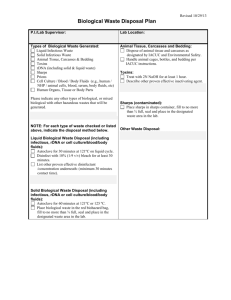
Butte School District PERSONNEL 5600P page 1 of 7 Bloodborne
advertisement

Butte School District 5600P page 1 of 7 PERSONNEL Bloodborne Pathogen-Exposure Control Exposure Determination The Montana Safety Act requires employers to perform an exposure determination concerning which employees may incur occupational exposure to blood or other potentially infectious materials. The exposure determination is made without regard to use of personal protective equipment (i.e., employees are considered to be exposed, even if they wear personal protective equipment). This exposure determination is required to list all job classifications in which all employees may incur such occupational exposure, regardless of frequency. The District has determined the following job classifications to be in this category: Nurses Trainers Monitors and teachers assigned to special education self-contained classrooms Elementary/middle school secretaries Special education bus monitors Job Classification and Task/Procedure Nurses Trainers; Special Education Self-Contained Injections Classroom Teachers and Monitors; Secretaries Venipuncture Biohazardous-waste handling CPR/First Aid procedures and instruction CPR/First Aid procedures and instruction Emergency response and procedures Emergency response and procedures Catheterizations Compliance Methods Universal Precautions Universal precautions will be observed by all District personnel, in order to prevent contact with blood or other potentially infectious materials. According to the concept of universal precautions, all blood and other potentially infectious material is to be treated as if it were infectious. Work Practice Controls 1. Hand Washing. Employees are to wash hands and other skin with soap and water and flush mucous membranes with water immediately or as soon as possible following contact of those body areas with blood or any potentially infectious material. 5600P page 2 of 7 a. Where hand-washing facilities are not available, an antiseptic cleaner and a clean cloth/paper towel or antiseptic towelettes will be provided. These are available for staff use through the District warehouse. If antiseptic hand cleaners or towelettes are used, the contaminated area is to be washed with soap and running water as soon as possible after exposure. b. Employees are to wash their hands with soap and water as soon as feasible after removal of gloves. 2. Needles. Contaminated needles and other contaminated sharps will not be broken, bent, or recapped. 3. Containers for Reusable Sharps. Contaminated sharps are to be placed into appropriate sharps containers as soon possible after use. Sharps containers are puncture resistant, labeled with a bioharzard label, and leakproof. These containers are located in the health room at each school. 4. Work Area Restrictions. In areas with a reasonable likelihood of exposure to blood or other potentially infectious materials, employees are not to eat, drink, apply cosmetics or lip balm, smoke, or handle contact lenses. 5. Housekeeping. Any contaminated area will be cleaned and decontaminated after each contamination. Decontamination will be accomplished utilizing 1:10 household bleach solution or other EPA-registered germicide. All contaminated work surfaces will be decontaminated after completion of procedures and immediately or as soon as reasonably feasible after any spill of blood or other potentially infectious materials, as well as at the end of the work shift if the surface may have become contaminated since the last cleaning. All bins, pails, cans, and similar receptacles will be inspected and decontaminated on a regular basis. Broken glassware which may be contaminated will not be picked up directly with the hands. Use a broom and dust pan to pick up glass; decontaminate the area with a bleach solution and paper towels. Dispose in plastic, puncture-resistant container and place in a biohazard bag. A sharps container may be substituted. 6. Regulated Waste. All contaminated sharps will be discarded as soon as feasible into sharps containers, which will be easily accessible to personnel and as close as feasible to the immediate area where sharps are used. The sharps containers will be closed immediately prior to removal from the area of use. Tape may be used to secure the lid but is not to be used as the lid itself. If leakage is possible, the sharps container will be placed in a secondary container which is able to be closed to prevent leakage during handling, storage, transport, and shipping. The container will be labeled with a biohazard label. When using a sharps container with mail disposal service, follow 5600P page 3 of 7 the manufacturer’s instructions for repackaging and shipment. During handling, storage, transport, or shipping, if outside contamination of the regulated waste container occurs, it will be placed in a secondary container. Disposal of all regulated waste will be in accordance with applicable regulations. 7. Labels. Warning labels will be affixed to containers of regulated waste or other potentially infectious materials. 8. Personal Protective Equipment (PPE). Use of personal protective equipment is mandatory, when exposure to blood or other potentially infectious materials is anticipated. All PPE will be provided without cost to employees. Gloves will be worn, when it is reasonably anticipated employees will have contact with blood, other potentially infectious materials, non-intact skin, and mucous membranes. Gloves will be available from the school nurse or can be ordered through the District warehouse. Gloves should be worn: a. When direct care of a student or coworker may involve contact with blood or other potentially infectious materials, non-intact skin, and mucous membranes. b. When contact with urine, feces, and respiratory secretions is anticipated. c. When changing a diaper or catheterizing a student. d. When providing mouth, nose, or tracheal care. e. If the caregiver has broken skin on the hands (even around the nails). f. When cleaning up spills of blood (e.g., nosebleeds) or body fluids and wastes and soiled supplies. Gloves should be disposed of after each use and should not be reused. Gloves are to be replaced as soon as torn or punctured, or when their ability to function as a barrier is compromised. Utility gloves may be decontaminated for reuse, provided the integrity of the glove is not compromised. Utility gloves will be discarded, if they are cracked, peeling, torn, punctured, exhibit other signs of deterioration, or when their ability to function as a barrier is compromised. Hepatitis B Vaccine Pre-exposure Hepatitis B vaccine will be offered through the District to all employees identified as having occupational exposure to blood or other potentially infectious materials (see Exposure Determination, above), at no charge to the employee, within ten (10) working days of their initial 5600P page 4 of 7 assignment to work. Employees identified as having occupational exposure, who decline Hepatitis B vaccine, will be required to sign a declination waiver for the Hepatitis B vaccine. The building administrator will assure the vaccine is offered and/or waivers are signed. School nursing staff will administer the vaccine. Employees who initially decline the vaccine but later wish to have it may have the vaccine provided at no cost, if they are in a position identified by the District as having occupational exposure to blood or other potentially infectious material. Post-Exposure Evaluation and Follow-Up 1. Minimize Exposure. In event of exposure, the employee is to immediately wash any exposed skin with soap and water and flush mucous membranes with water. 2. Exposure Incident Report. An employee who has had an exposure is to report such incident to the school nurse or, if the nurse is unavailable, the building administrator. The nurse will ascertain the source individual’s identity, arrange for testing of the source individual, and communicate with the physician evaluating the employee. An Exposure Incident Report (attached) will be completed by the employee. The report will be given by the employee to the evaluating physician, and a copy will be maintained in the employee’s confidential medical file, which will be maintained in the personnel office. Post-Exposure Medical Evaluation Immediately following reporting an exposure, the exposed employee will receive a confidential medical evaluation and follow-up conducted by a licensed physician or healthcare worker, at no cost to the employee. The evaluation must include: 1. Documentation of route of exposure and circumstances under which exposure occurred. 2. Identification of the source individual where possible and not prohibited by state or local law. (A state Safety Bureau representative will be contacted for help in learning current state and local laws.) 3. HBV and HIV blood-test results of the source individual as required, if feasible, and after consent is obtained, unless the source is known to be infected with HBV and/or HIV. If consent is not obtained, the nurse will document that legally required consent cannot be obtained. The exposed employee will be offered blood collection and/or testing. All required laboratory tests will be done by an accredited laboratory at no cost to the employee. The employee has the right to refuse either or both. However, if the exposed employee gives consent for blood 5600P page 5 of 7 collection but not for HIV testing, the blood is kept for ninety (90) days. During this time the employee may choose to have the sample tested. The employee will be offered appropriate post-exposure prophylaxis, including immune globulin for Hepatitis B. Recommendations of an evaluating physician familiar with current CDC guidelines on post-exposure prophylaxis for HIV are followed in the event of HIV exposure. Results of the source individual’s blood test will be made available to the exposed individual, in accordance with state and local guidelines. Counseling and evaluation of any reported illnesses are provided at no charge to the exposed employee. Written Opinion of Healthcare Professional A written opinion by the evaluating healthcare professional, stating that the exposed employee has been informed of results of the evaluation and about any exposure-related conditions which will need further evaluation and treatment, is included in the employee’s confidential medical record. The written opinion is made available to the exposed person within fifteen (15) days of completion of the evaluation. The written opinion for HBV vaccination will be limited to whether the HBV vaccination series is indicated, and whether the employee has received that vaccination series. The written opinion for post-exposure follow-up will be limited to the following information: 1. A statement that the employee has been informed of results of the evaluation. 2. A statement that the employee has been told about any medical conditions resulting from the exposure, which require further evaluation or treatment. NOTE: All other findings or diagnoses remain confidential and are not to be included in the written report. Record Keeping The following record-keeping procedures will be followed: The medical records are kept at least thirty (30) years after the person leaves employment. Medical records are confidential and are kept for all employees with occupational exposure and must include: 5600P page 6 of 7 1. Employee’s name and social security number. 2. Hepatitis B vaccination status, including dates of vaccinations, records relating to employee’s ability to receive the vaccine, and signed declination form where applicable. 3. All information given to evaluating healthcare professional in the event of an exposure incident. 4. Copy of evaluator’s opinion. Written permission is required for access to the employee’s medical record. Confidential medical records are kept in the personnel office. Employee medical records are available upon request to the Department of Labor and Industry, Safety Bureau. Training Training will be conducted for all employees prior to initial assignment to tasks where occupational exposure may occur. At a minimum training must include: 1. Information regarding the standard and an explanation of its requirements; 2. General explanation of epidemiology and symptoms of bloodborne diseases; 3. Explanation of modes of transmission of bloodborne diseases; 4. Explanation of employer’s exposure-control plan or policy and the means by which the employee can obtain a copy of the written policy; 5. Explanation of appropriate methods for recognizing tasks and other activities that may involve exposure to blood and other potentially infectious materials; 6. Explanation of use and limitations of methods that will prevent or reduce exposure, including appropriate engineering controls, work practices, and personal protective equipment; 7. Information on types, location, proper use, and disposal of personal protective equipment; 8. Information on appropriate actions to take and persons to contact in an emergency involving blood or other potentially infectious materials; 9. Explanation of procedure to follow if an exposure occurs, including method of reporting the incident and the medical follow-up which will be made available. 5600P page 7 of 7 10. Information on the post-exposure evaluation and follow-up the employer is required to provide for the employee following an exposure incident; 11. Opportunity for interactive questions and answers with the person conducting the training. Bloodborne Pathogen – Post-Exposure Protocol The following sites are designated to provide employees with post-exposure evaluation and treatment: St. James Healthcare Emergency Room or Private physician The Employee MUST: Report occupational exposure to the building administrator and/or immediate supervisor, within twenty-four (24) hours. Complete Accident Report and initiate Employee Occupational Exposure Incident Form. Go to a designated site listed above for treatment. (A post-exposure while on duty is considered a Workers’ Compensation case, if it meets the definition of an accident under the Workers’ Compensation statutes.) See Bloodborne Pathogen Exposure Control Plan for details. Procedure History: Promulgated on: 10/18/04 Revised on: