Tissue Repair, Cellular Growth, Fibrosis, & Wound Healing

MOD #82
Mon, 05/12/03, 10am
Dr. Mathew
J. Uxer for Matt Blackburn
Page 1 of 6
Structure and Function of Immunoglobins
Note that the post exam review has been moved to Wed, 2pm because Dr.
Eisenberg is out of town today.
Dr. Mathew is an associate professor of microbiology and has been teaching here for about 7 years. His research concentrates on natural killer cells. Be sure to ask him ask him questions if you have any.
I. Introduction
A. Objectives
Discuss the basic structural and functional features of Immunoglobulins
(Heavy and light chains, Fab, F(ab’)2, Fc, Hinge region, Ig domains, CDRs etc.)
Compare and contrast the effector functions of major isotypes of immunoglobulins
Discuss the following: Antigen binding site; Affinity and Avidity
B. Immunoglobins (Ig) or Antibodies
The terms immunoglobin and antibody refer to the same thing.
Antigen vs. Immunogen o Antigen—any molecule that will bind to an antibody. Does not necessarily illicit an immune response o Immunogen—a molecule that will bind to an antibody and illicit an immune response. o So, all immunogens are antigens but not all antigens are immunogens
Serum Glycoproteins o level increased when an animal is exposed to antigen or immunogen o Ig are glycoproteins—primarily a carbon chain with a glucose moiety added onto it.
Expressed on B cells as the B cell receptor. Once the antigen binds to this, the cell becomes a plasma cell.
Secreted by Antibody Forming Cells (AFC) or plasma cells which are terminally differentiated B cells.
All Igs are bifunctional o One region of the molecule (Fab) binds antigen whereas the other region
(Fc) is involved in effector function. o This does not mean they only have 2 functions. o All Igs do 2 common functions:
1.
Bond to antigen
2.
Carry out an effector function—decide what to do with the antigen
II. Structure of Immunoglobins
A. Basic Structure
Igs are made of 4 peptide chains: 2 larger chains called heavy (H) chains and 2 smaller chains called light (L) chains. Each are joined via disulfide bonds.
MOD #82
Mon, 05/12/03, 10am
Dr. Mathew
J. Uxer for Matt Blackburn
Page 2 of 6
The Ig as a whole unit of 4 peptide chains is referred to as a monomer. 2 Igs associate to form a dimer, 3 to make a trimer, and so on.
All membrane bound forms of antibodies are tetramers of two separate polypeptide chains.
One chain is of approximately 50 kDa, and is termed the Heavy chain or H chain, and the other of 25 kDa is termed the Light chain or L chain.
Ig weighs about 150kDa. The weight does vary depending on the level of glycosylation.
In all antibodies there are only two types of light chains: lambda (
There can only be 1 type of light chain in an individual Ig.
There are five major heavy chains (μ, γ, δ, α, and ε) which give rise to the five functional classes of Igs: μ—IgM, γ—IgG, δ—IgD, α—IgA and ε—
IgE.
The heavy and light chains are held together by disulfide bonds and noncovalent interactions
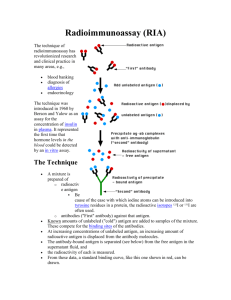
B. Enzymatic Digestion of Immunoglobulins
Antigen binds here
Purified IgG could be cleaved
Fab— by proteolytic enzyme papain binds 1 into three fragments by breaking the disulfide bond.
Two fragments are identical and contain antigen binding
F(ab’)2 binds 2 antigens activity, and these are termed
Fab (Fragment antigen binding) fragments.
The other fragment is readily crystallizable and therefore called Fc fragment.
Pepsin cleaves an identical immunoglobulin to a fragment twice the size of
Fab and is called F(ab’)2 and several other fragments, the largest one called pFc’.
Fab And F(ab’)2 Fragments o Fab is monovalent whereas F(ab’)2 is bivalent. A monvalent Fab’ can be formed by treating F(ab’)2 with reducing agents such as mercaptoethanol o Both Fab and F(ab’)2 can bind antigen. Fab binds only 1 antigen; whereas, F(ab’)2 binds 2 antigens. o Only F(ab’)2 will result in an immunoprecipitation since it binds 2 antigens.
C. Immunoglobulin Domains
MOD #82
Mon, 05/12/03, 10am
Dr. Mathew
J. Uxer for Matt Blackburn
Page 3 of 6
The heavy and light chains can be divided into domains on the basis of sequence similarity.
An Ig domain consists of about 110 amino acids and contain two cysteine residues which form intrachain disulfide bonds
The amino terminal domains of the heavy and light chains are variable and are referred to as variable domains.
The remaining domains are constant.
Each light chain has one variable domain, VL and one constant domain, CL.
Each Heavy chain has one variable domain, VH and at least three constant domains, CH1, CH2 and CH3. Cγ1 is the constant of domain 2 on heavy chain γ.
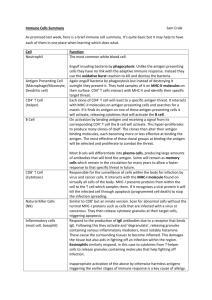
STRUCTURE OF IgG
Light Chain
Hinge Region
Heavy Chains
Variable Region
Constant Region
D. Hinge Region
A segment of 10 to 15 amino acid residues between the constant regions 1 &
2 on the heavy chain (CH1 and CH2 domain) is known as the hinge region
The hinge region is rich in cysteine and proline residues. However this region allows flexibility (Segmental flexibility) so that antibodies can bind repetitive antigens simultaneously
The hinge allows the 2 antigen binding ends of the Ig to move from ~0° to
>90° to better bind antigens.
Hinge region is found in α, γ, and δ chains, but absent in
μ and ε chains. So, IgM and IgE do not have a hinge. Instead they have a CH4 domain.
E. Hypervariable Regions (CDRs)
MOD #82
Mon, 05/12/03, 10am
Dr. Mathew
J. Uxer for Matt Blackburn
Page 4 of 6
When you plot variability (the heavy chain variable region or the light chain variable region) against amino acid residue number of various Igs (Wu and
Kabat plot) three distinct hypervariable regions (HV1, HV2 and HV3) are found in the variable domains.
The hypervariable residues of the light and heavy chain form the antigen binding site and interact with antigens on the basis of structural complementarity and are therefore known as Complementarity
Determining Regions (CDRs) and are denoted by CDR1, CDR2 and CDR3.
The most variable part is in the HV3 (CDR3) region
The hypervariable regions are surrounded by less variable regions, termed
Framework Regions (FR1, FR2, FR3 and FR4). These provide support for the hypervariable regions
III. Antigen Binding
A. Antigen Binding Site
The antigen binding site is formed by the VL and VH domains.
When an antigen is encountered, the antibody folds bringing the CDRs to the surface.
Pairing of the heavy and light chains brings together the hypervariable regions (CDRs or HVs) to create a single hypervariable surface that forms the antigen binding site
The antigen binding site can accommodate 6 or 7 carbohydrate residues of an oligosaccharide or 7 to 11 amino acid residues of a polypeptide.
The antibody only recognizes 7 – 11 residues, the antigen determinate, present on the surface of the bacterial glycoprotein and binds to these.
This is like a T-cell in that 7 – 11 amino acid residues are recognized, but it is different because the antibody can recognize these residues while they are a part of the antigen. The antigen must be cleaved, antigen presentation, in order for the T-cell to recognize the residues
B. Antigen: Antibody Interactions
Ag:Ab interaction is always reversible because its bonding is noncovalent
The forces involved in Ag:Ab interactions are all weak forces: Electrostatic forces, Hydrogen bonds, Van der Waals forces and Hydrophobic forces
Ag:Ab interactions do not involve covalent bonds
IV. Immunoglobulin Isotypes
There are five major classes (isotypes) of Igs: IgG, IgM, IgA, IgD and IgE
All have 2 common functions:
1.
Recognize and bind the antigen
2.
Effector function—1 example is complement activation in order to generate complement proteins to kill the bacteria.
A.
IgG
In normal serum IgG is the major class - 75% of Ig.
MOD #82
Mon, 05/12/03, 10am
Dr. Mathew
J. Uxer for Matt Blackburn
Page 5 of 6
There are four subclasses of IgG: IgG1, IgG2, IgG3 and IgG4
(70%,20%,8% and 2% respectively). All subclasses except IgG4 activate complement.
IgG can cross placenta and thus provide passive immunity to new born.
This lasts for about 6 – 9 months. So, you usually see kids get sick a lot from 6 months to a year because the IgG has worn off and the infant’s immune system is just starting to kick in at 1 year of age.
Because IgG can cross the placenta, it is responsible for Hemolytic
Disease of Newborns. o Blood groups have antigens. + is the antigen for Rh. o Scenario: Dad is Rh+, Mom is Rh-, and fetus is Rh+. During the 1 st pregnancy and delivery, there is no problem. The kid’s blood and the mom’s blood do mix, however. This causes Mom to make anti Rh antibodies (IgG+) since her blood is Rh-. Now, in the following pregnancies, there can be a problem if the fetus is Rh+. Mom’s antibodies for Rh+ can cross the placenta and attack the fetus which is seen as a foreign invader since its blood is different than Mom’s. Drugs can be given to the mom to prevent this from occurring, to get rid of her anti-Rh+ antibodies.
B.
Immunoglobulin M (IgM):
IgM is the first antibody produced in a [any] humoral immune response
IgM is a pentamer (5 IgM monomers joined together) (H2L2)5 and has 10 active antigen binding sites. This allows it to bind to the antigen with a stronger bond due to the 10 binding sites.
Each IgM contains a joining piece (J chain, a polypeptide) which is disulfide linked to the molecule. The J chain joins the 5 IgMs together.
IgM activates complement.
IgM is found on the surface of B cells. Once an antigen is encountered, the
B cell can switch the type of Ig on its surface from IgM to another Ig. This is called Ig class switching.
IgM is high in the early stages of infection, the 1 st
2 -3 weeks.
C.
Immunoglobulin A (IgA):
IgA is the principal isotypes in mucosal secretions.
IgA is present in high levels in the initial breast secretions (colostrum) and provide protection to new born infants against bacteria in the gut. This is why it is important to breast feed early.
There are two subclasses of IgA: IgA1 and IgA2.
Note that if you see C1, C2A, C2B, C3 you are looking at Igs for a mouse.
The corresponding Igs for humans are C1, C2, C3, C4.
IgA exist in both monomeric and dimeric forms. Dimeric form has the J chain.
Secretory IgA is bound with another protein called secretory piece (which is derived from the poly-Ig receptor on epithelial cells) which protect dimeric
MOD #82
Mon, 05/12/03, 10am
Dr. Mathew
J. Uxer for Matt Blackburn
Page 6 of 6
IgA from proteolytic cleavage. This gives more stability to secretory IgA dimer.
D.
Immunoglobulin D (IgD):
Low concentration in serum (<1%)
Found on surface of B cells before they interact with an antigen
The function is not well characterized and not known.
Before a B-cell interacts with an antigen, both IgM and IgD are expressed on its surface. This is the only time a B-cell presents with 2 different classes simultaneously. The same B cell can change the type of
Ig it expresses by turning off the 1 st
type and switching on another type.
E.
Immunoglobulin E (IgE):
IgE is the lowest concentration in serum
IgE is the main mediator of allergic reactions—hypersensitivity reactions, anaphylaxis.
IgE binds to receptors on mast cells and basophils and release mediators
(such as histamines) upon binding of antigen.
An antigen binds and a cross link is formed with the mast cell causing the allergic reaction.
Allergen → binds IgE → binds to receptor on mast cell → release of mediators from mast cell → allergic reaction
Principle of the allergy shot: make the body generate more IgG for a particular allergen so that when the allergen is encountered there’s a shift from the hypersensitivity reaction to a different response. If the [IgG] is increased, then it will arrive at the allergen and bind to it before the IgE, which is in lower concentration, arrives. This averts the hypersensitivity reaction.
F. Summary of Immunoglobulin Functions
Causes hemolytic disease of newborn
Hypersensitivity reaction