The TBS implant design and surgical protocol for quantifying the
advertisement

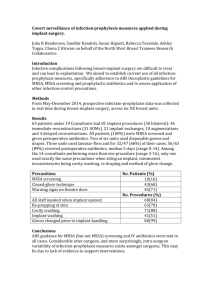
Short title: Interfacial biochemical bond strength An in vivo method for quantifying the interfacial biochemical bond strength of bone implants Short title: Interfacial biochemical bond strength Young-Taeg Sul*, DDS, PhD1, Carina Johansson, PhD2 and Tomas Albrektsson, MD, PhD3 1 Associate professor, Department of Biomaterials/Handicap Research, Institute for Clinical Sciences, Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden. 2 Professor, School of Health and Medical Sciences, Department of Clinical Medicine, Örebro University, Örebro, Sweden. 3 Professor, Department of Biomaterials/Handicap Research, Institute for Clinical Sciences, Gothenburg University, Gothenburg, Sweden. Corresponding author information Dr. Young-Taeg Sul. Mailing address: Department of Biomaterials/Handicap Research, Institute for Clinical Sciences, The Sahlgrenska Academy, Gothenburg University, Box 412, SE 405 30, Gothenburg, Sweden. Tel: +46 31 786 2950 Fax: +46 31 786 2941 1 Short title: Interfacial biochemical bond strength SUPPLEMENTARY METHODS Design details of the TBS implant device All dimensions of the TBS components were detailed in Supplementary Fig. 1. The disc implant (Supplementary Fig. 1b) had a 45 degree and a 0.5 mm long bevelended functional surface of 8 mm diameter and a cylindrical transducer with a 2 mm hole for connecting to the sample holder. To reduce shear and tilting forces, the pin hole was tapered and connected by a pin and pinhole connection. The housing frame (Supplementary Fig. 1c) consisted of the bevel seat for the fit of the disc implant, a cylindrical lip at the bottom, two arms, and necks and holes for the retention screw. The cylindrical lip was designed to stabilize the TBS implant device by pressfitting into the bone bed at the same dimension of 10 mm diameter, 0.5 mm depth. The cover screw (Supplementary Fig. 1d) fit in the chamber of the housing and held the disc implant in a desired bevel seat position through point contact with the conical apex of the cylindrical transducer of 2 Short title: Interfacial biochemical bond strength the disc implant. The retention screw (Supplementary Fig. 1e) was 3.3 mm wide and 7 mm long, with 10 tapered threads for obtaining good retention of the TBS implant device in bone. For possible adaptation of the TBS frame to individual variance of the tibia contour, we designed a notched neck along the arm and a tolerance between the retention screw and screw hole (3.3 mm vs. 4.0 mm). Surgical protocol for stress-free stabilization of the TBS implant device on bone The animals were anesthetized with intramuscular injections of fentanyl and fluanison (Hypnorm Vet, Janssen, Saunderton, England) of 0.5 ml per kg body weight and intraperitoneal injections of diazepam (Valium, Roche, France) of 2.5 mg per animal. The skin and fascial layers were opened separately. The periosteal layer was gently pulled away from the surgical area. To prepare the precise bone bed for stabilizing the TBS implant device, we used a custom-made 3 flute end mill set of 2, 4, 6, 8 and 10 mm diameters with a stopper of 0.5 mm height and a central guide pin of 2 mm diameter. The desired position of the TBS device was marked on the tibia cortical bone plate with assistance of a surgical guide template, followed by twist 3 Short title: Interfacial biochemical bond strength drills to make a 2.0 mm bone hole for the functional disc implant and the 3.0 mm holes for the fixation screws. When consecutively milling, care must be taken to ensure the bone bed floor is flattened and parallel to the tibia contour without perforating the floor of the bone bed through the marrow cavity as the tibia cortex of the surgical site of interest was thin, approximately 1.8 mm to 2.0 mm. The drill stopper and central guide pin allowed the surgeon to prepare the precise bone bed for stabilizing the TBS implant device. The housing was positioned by fitting the cylindrical lip on the flat-bottomed bone, stabilizing the TBS implant device. The disc implant was fitted into the bevel seat of the housing by screwing down the cover screw. Alternatively, the housing, disc implant, and cover screw can be put together prior to surgery. This can save operation time and confirm the high precision of the bevel seat fitting between the disc implant and the housing. Stabilization of the TBS device was finished with final adjustment of the retention screws. The skin and fascial layers were closed but the periosteal layer was not resutured. During all surgical drilling and milling, low rotary drill speeds and saline cooling were used. After surgery, the animals were kept in separate cages and allowed full weight-bearing. The animals were sacrificed by intravenous injections of an overdose of Pentobarbital® (Apoteksbolaget, Uppsala, Sweden). Supplementary captions Supplementary Figure 1. Detailed dimensions of the TBS implant devices in vertical crosssection view taken along the middle line. (a) Top and cross-section view of TBS implant 4 Short title: Interfacial biochemical bond strength assembly, (b) top and cross-section view of the housing frame (a-①), (c) top and lateral view of functional disc implant (a-②), (d) top and cross-section view of cover screw (a-③), and (e) top and lateral view of retention screw (a-④). Supplementary Figure 2. Test set-up: (a) test machine, (b) a three-dimensionally adjustable sample mount at the micrometer scale, and (c) the functional disc implant coupled to the load transducer after measurement. The test machine was calibrated with a measurement accuracy of better than 0.4 % using a load cell of 10 N at a constant load rate of 10 mm/min. 5 Short title: Interfacial biochemical bond strength Supplementary Table 1. Interfacial bond strength of the implants, MPa. 6