HOD by Dr. Angles - Win`Weim Weimaraners
advertisement

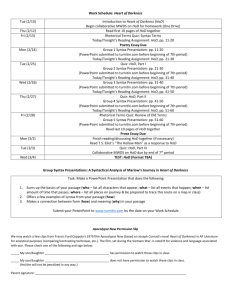
TITLE: “IDENTIFICATION OF A GENETIC MARKER FOR HYPERTROPHIC OSTEODYSTROPHY IN THE WEIMARANER” PRINCIPAL INVESTIGATOR: Dr. John M. Angles BVSc, BSc (Vet), DACVIM, DECVIM-CA Ralston Purina Lecturer in Genetics and Medicine Faculty of Veterinary Medicine University College Dublin Ballsbridge, Co Dublin 4 IRELAND Phone: +353-1-668-7988, extension 2632 Fax: +353-1-667-5401 Email: <john.angles@ucd.ie> CO-INVESTIGATORS: Dr. Thomas Famula, PhD Professor, Department of Animal Science University of California, Davis Dr. Niels C. Pedersen, DVM, PhD Director, Veterinary Genetics Laboratory University of California, Davis SUMMARY OF RESEARCH: Hypertrophic Osteodystrophy (HOD) is a common disease of the rapidly growing dogs of the large and giant pure-breeds. Breeds found to be at higher risk for HOD include the Great Dane, Weimaraner, Irish Setter and Irish Wolfhound. For the past 2 years we have been investigating the genetics of HOD in the Weimaraner breed under AKC (CHF) grant # 1628. The disease is particularly severe in the Weimaraner, with systemic manifestations including fever and multiple body organ inflammation. Males are equally affected as females, and the age of onset is typically 8 to 16 weeks of age. Our data indicate that HOD in the Weimaraner is a genetic disease, with a heritability of 0.35 and an autosomal recessive mode of inheritance. Unfortunately, to date there is no method available to detect carriers other than by test matings. We estimate that some 30% of the breed are carriers for this debilitating disease. DNA samples from at least 10 separate 3-4 generation families with HOD have been collected, and our aim is to perform a genetic marker study to identify a marker for susceptibility to HOD in the Weimaraner breed. It is anticipated that with an appropriate DNA marker, rapid progress can be made to decrease the prevalence of this disease in the breed. SIGNIFICANCE OF RESEARCH: Hypertrophic Osteodystrophy (HOD) is a debilitating disease of large pure-breeds of dog. HOD in the Weimaraner is a particularly severe disease, with significant morbidity and mortality documented. Discovery of a genetic marker for HOD in the Weimaraner will have important ramifications for the breed. For the first time, breeders will be able to perform selective breeding to decrease the prevalence of carriers and thereby disease in the breed. Twenty years of selection based on test matings has unfortunately not been able to achieve this objective. The Great Dane and Irish Setter breeds also have a severe form of HOD disease, and an understanding of the genetic basis of the disease in the Weimaraner is likely to assist studies in progress for these breeds. BACKGROUND OF PROPOSED RESEARCH: Hypertrophic Osteodystrophy (HOD) is a common disease of rapidly growing, large to giant pure-breeds of dog. We recently performed a retrospective analysis of the Veterinary Medical Data Base (VMDB) at Purdue, and found that the Weimaraner had a relative risk of 16.4 for developing HOD when compared to the entire population [Final report, AKC (CHF) grant #1628]. Other breeds also at high risk included the Great Dane (23.1), Irish Setter (10.4) and Irish Wolfhound (11.4). Most dogs present for the pain and lameness associated with swelling of the metaphyses of the long bones. Fever and anorexia are also commonly associated with clinical HOD (Muir, 1996). The Weimaraner breed unfortunately has a severe form of HOD disease compared to most breeds, with systemic manifestations including fever and multiple body organ inflammation (Abeles, 1999; Angles and Pedersen, 2000). Diagnosis of HOD relies on the typical signalment, clinical signs, and presence of pathognomic radiographic changes in the metaphysis. The cause of canine HOD remains unknown, with earlier speculations of a vitamin C deficiency (Meier, 1957; Holmes, 1962) or over-nutrition (Riser, 1965), discounted in more recent times (Grondalen, 1976). There is mounting evidence that viral infection may be important in the disease, with Distemper virus detected in the metaphyses of dogs with HOD (Mee, 1992; Baumgartner, 1995; Mee, 1993). However, to our knowledge no one has been able to experimentally reproduce HOD with Distemper Virus infection, suggesting that there are other important factors in the pathogenesis of this disease. An important finding of AKC (CHF) grant # 1628 was an association between HOD disease and recent vaccination. Approximately 70% of the Weimaraners diagnosed with HOD had received a recent multi-valent vaccine within 1-2 weeks of disease onset. Most of these had been vaccinated within 2-3 days of disease onset (Angles and Pedersen, 2000). It is important to note that there have been Weimaraners with HOD not receiving vaccines within the previous 3 weeks, indicating that the vaccination is only one trigger for HOD disease on a susceptible genetic background. An epidemiological study is in progress to further define the role and type of vaccines involved in causation of HOD in the Weimaraner. The mode of inheritance of HOD in most breeds of dog has not been reported, but there is an obvious breed predisposition suggesting that genetic factors play an important role. This has been reinforced in the Weimaraner breed, with a litter of four affected Weimaraner puppies (Woodard, 1982), and a different litter of four affected puppies and a daughter (Grondalen, 1976) reported in the literature. Our data indicate that HOD in the Weimaraner is a genetic disease, with a heritability of 0.35 and an autosomal recessive mode of inheritance (Angles and Famula, 2000). It is not possible to detect carriers at present other than by test matings, as there are no known biochemical or conformational traits to indicate their carrier status. Based on retrospective mating analysis, over 100 carrier sires and dams are known to one of the investigators, and we estimate that some 30% of the breed are carriers for this debilitating disease. PRELIMINARY WORK: The genetics of Hypertrophic osteodystrophy (HOD) in the Weimaraner have been studied in AKC (CHF) grant # 1628. The mode of inheritance of HOD in the Weimaraner is autosomal recessive based on retrospective analysis of over 100 HOD affected Weimaraners and their extended families. We currently have available DNA samples for 70 HOD affected Weimaraners, and have developed 10 3-4 generation informative families with HOD segregating for full linkage analysis. DNA samples are stored at the University College Dublin and are freely available for collaboration with other investigators by petition to the principal investigator. Preliminary assessment of markers for the full linkage study was completed in AKC (CHF) grant # 1628 at the Veterinary Genetics Laboratory, University of California, Davis. 100 unrelated Weimaraner samples derived from the 1200 samples available in the DNA-disease database were evaluated on the UCDavis multiplexed linkage sets 1 to 7. These linkage sets have 14 individual markers per set and have been designed to give 15-20cM coverage of the canine chromosomes. Results of this analysis were encouraging, as this population had been considered highly inbred prior to DNA analysis. Markers AHT132, CO3.877, CPH03, CPH16, FH2293, FH2361, LEI005, LEI007, Wilms-TF performed poorly in this population of dogs, such that reliable data was only available for very few of the 100 dogs. Further, markers AHT136, AHTk211, AHTk292, CO1.424, CO6.636, CO9.173, CO9.250, CXX391, CXX608, FH2004, FH2148, FH2328, FH2356, LEI004, LEI006, PEZ02, PEZ05, RVC1, and VIASD10 had PIC values <0.3, which may limit their usefulness for a subsequent linkage study. It is proposed to replace these markers with doubtful information for a linkage study with markers in the same region to give adequate chromosomal coverage. Ten informative families are available for full linkage analysis to date, with several more in advanced development (available for review on request to principal investigator). One of the reasons for performing the preliminary marker analysis prior to using these samples was that for some family members there is limited DNA available and it is too precious to waste on validating the markers. We intend to make the marker information for the breed available to other investigators on a website to facilitate like projects in the future. SPECIFIC OBJECTIVES OF THE STUDY: Our primary objective is to identify a genetic marker for Hypertrophic Osteodystrophy (HOD) in the Weimaraner breed. This will allow breeders to adopt selective breeding practices to decrease the number of carriers and thereby reduce the prevalence of this debilitating disease in the Weimaraner. Knowledge of the physical location of the marker/s will facilitate studies examining the actual cause for this disease, and may have application to other breeds that suffer from HOD. RESEARCH DESIGN: DNA samples for the ten informative families are already available for marker analysis at the University College Dublin. Three additional families are under development, and may be introduced into the study if sufficiently informative. Based on pedigree and preliminary marker analysis, we believe that a classical linkage study will be the appropriate approach to analyzing these families. Markers available from either the Research Genetics, University of Rennes, Animal Health Trust or UCDavis will be selected to replace the markers that were not sufficiently informative in the preliminary study. Our aim will be to attain a 10-15cM coverage of the chromosomes to look for linkage. Any areas that look like there may be linkage will be further saturated with available markers. The multiplex panels will be run at the Veterinary Genetics Laboratory at University of California, Davis, and markers added to supplement these would be run separately at University College Dublin. Both facilities have in-house ABI 377 DNA sequencers and full range of molecular biology equipment. We intend to run the markers across three pooled sets of samples initially, i.e. known affected, known carriers and known clears. This will allow us to increase the marker saturation at an early stage in areas of interest. The complete families will then be run for full linkage analysis to confirm results. Statistical analysis will be performed using algorithms designed by Dr. Tom Famula at the University of California, Davis. These algorithms integrate previous pedigree information available on the DNA-disease database at University College Dublin with the allele information available of reach of the markers in the linkage study. These techniques have already been validated using for linkage analysis for seizures in the Belgian Tervuren. EXPECTED OUTCOMES/POTENTIAL APPLICATIONS: The primary objective in the short term is to discover a genetic marker that will be useful for the Weimaraner breeders to perform selective breeding to remove carriers from the breed pool and thereby reduce the prevalence of HOD in the breed. The principal investigator has already presented several seminars to the Weimaraner Club of America on the appropriate use of these marker tests and their deficiencies. Availability of sound genetic counselling will be important to the successful use of any marker detected by this study. Using syntenic maps, candidate genes for HOD disease will be assessed based on the physical location of the marker on the chromosome. Our long term aim is to understand the pathophysiology of the disease and to use this knowledge to assist other breeds such as the Great Dane and Irish Setter that have similar severe forms of HOD. REFERENCES: 1) Abeles, V, Harrus, S, Angles, JM, Shalev, G, Aizenberg, I, Peres, Y and Aroch, I (1999) Hypertrophic osteodystrophy in six weimaraner puppies associated with systemic signs. Vet Record, 145: 130-134 2) Angles, JM and Pedersen, NC (2000) Hypertrophic Osteodystrophy (HOD) in the Weimaraner: 60 cases. JAAHA (in preparation) 3) Angles, JM and Famula, TR (2000) Genetics of Hypertrophic Osteodystrophy (HOD) in the Weimaraner. JVIM (in preparation) 4) Baumgartner, W, Boyce, RW, Alldinger, S, Axthelm, MK, Weisbrode, SE, Krakowka, S and Gaedke, K (1995) Metaphyseal bone lesions in young dogs with systemic distemper virus infection. Vet Microbiol, 44: 201-209 5) Breur, GJ, Austin CC (1997) Breed predilections for developmental orthopedic diseases in the dog. AKC Molecular genetics and Canine Health Conference 1997, St. Louis, MO, Poster #5, pp95-96 6) Dougherty, SA, Center, SA, Shaw, EE and Erb, HA (1991) Juvenile-onset polyarthritis syndrome in Akitas. JAVMA, 198 (5): 849-856 7) Grondalen, J (1976) Metaphyseal osteopathy (hypertrophic osteodystrophy) in growing dogs. A clinical study. JSAP, 17: 721-735 8) Holmes, JR (1962) Suspected skeletal scurvy in the dog. Vet Rec, 74: 801-813 9) Meier, H, Clark, ST, Schnelle, GB, Will, DH (1957) Hypertrophic osteodystrophy associated with disturbance of vitamin C synthesis in dogs. JAVMA, 130: 483-494 10) Mee, AP, Gordon, MT, May, C, Bennett, D, Anderson, DC and Sharpe, PT (1993) Canine Distemper Virus Transcripts Detected in the Bone Cells of Dogs with Metaphyseal Osteopathy. Bone, 14: 59-67 11) Mee, AP, Webber, DM, May, C, Bennett, D, Sharpe, PT and Anderson, DC (1993) Detection of Canine Distemper Virus in Bone Cells in the Metaphyses of Distemper-infected Dogs. J Bone and Mineral Research, 7 (7): 829-834 12) Muir, P, Dubielzig, RR, Johnson, KA, Shelton, GD (1996) Hypertrophic osteodystrophy and calvarial hyperostosis. CCEPV, 18(2): 143-151 13) Parker, WM and Foster, RA (1996) Cutaneous vasculitis in five Jack Russell Terriers. Vet Dermatology, 7: 109-115 14) Riser, WH, Shirer, JF (1965) Normal and abnormal growth of the distal foreleg in large and giant dogs. J Am Vet Radiol Soc, 6: 50-64 15) Scott-Moncrieff, JCR, Snyder, PW, Glickman, LT, Davis, EL and Felsburg, PJ (1992) Systemic necrotizing vasculitis in nine young Beagles. JAVMA, 201 (10): 1553-1558 16) Snyder, PW, Kazacos, EA, Scott-Moncrieff, JC, HogenEsch, H, Carlton, WW, Glickman, LT and Felsburg, PJ (1995) Pathological Features of naturally Occurring Juvenile Polyarteritis in Beagle Dogs. Vet Pathol, 32: 337-345 17) Sorensen, D. A., Andersen, S., Gianola, D., and Korsgaard, I. 1995. Bayesian inference in threshold models using Gibbs sampling. Genetique, Seletion, Evolution. 27:229-249 18) Woodard, JC (1982) Canine hypertrophic osteodystrophy, a study of the spontaneous disease in littermates. Vet Pathol, 19: 337-354 BIOGRAPHICAL SKETCH of JOHN M. ANGLES: Education: 2000 Diplomate Companion Animal, ECVIM 1999 PhD Candidacy, University of California, Davis 1998 Diplomate Small Animal Medicine, ACVIM 1994-1996 Residency Small Animal Medicine, University of California, Davis 1993 MVStudies Murdoch University, Australia 1993 MACVSc Canine Medicine 1988 BVSc Honors Class 1, University of Sydney, Australia 1987 BSc(Vet) Veterinary Physiology, University of Sydney, Australia Honors, Awards: 1999 Phi Sigma Society, Gamma Delta Society 1997 Clinical Research Fellowship in Immunogenetics, University of California, Davis 1996 Postdoctoral Clinical Research Fellowship in Genetics, Center for Companion Animal Health, University of California, Davis 1988 Martin McIllwraith Scholarship, University of Sydney, Australia 1986 Selby Scientific Foundation Scholarship in Ultrasonography, University of Sydney 1984 Harrie Barrett Bursary, University of Sydney, Australia 1984 Prize for Biochemistry I and II and Veterinary Physiology I Auxillary to the Australian Veterinary Association (NSW State Division) Research and Professional Experience: 1999-Pres. Ralston Purina Lecturer in Small Animal Medicine and Genetics, Faculty of Veterinary Medicine, University College Dublin, Ireland 1998- Pres. Member of the ISAG DLA Nomenclature Committee 1996-1999 Postdoctoral Researcher, University of California, Davis 1994-1996 Resident, Small Animal Medicine, Veterinary Medical Teaching Hospital, University of California, Davis 1990-1994 Associate, Small Animal Medicine, Canberra Veterinary Hospital, Australia Publications: 2000 Ollier, WER, Kennedy, LJ, Thomson, W, Barnes, A, Bennett, D, Angles, JM, et al Rheumatoid Arthritis in both dogs and human is associated with a shared MHC epitope. J Experimental Medicine (accepted) 1999 Abeles, V, Harrus, S, Angles, JM, Shalev, G, Aizenberg, I, Peres, Y and Aroch, I Hypertrophic osteodystrophy in six weimaraner puppies associated with systemic signs. Vet Record, 145: 130-134 1997 Angles JM, Feldman EC, Nelson RW and Feldman MS Use of Urine Cortisol: Creatinine Ratio versus Adrenocorticotrophic Hormone Stimulation Testing for Monitoring Mitotane Treatment of Pituitary-dependent Hyperadrenocorticism, JAVMA 211 (8): 1002-1004 1990 Wood, AKW, McCarthy, PH and Angles, JM Ultrasonographic-Anatomical Correlation and Imaging Protocol for the Spleen in Anesthetized Dogs, Am J Vet Research, 51 (9): 1433-1438 1990 Angles JM, Walsh DA, Li K, Barnett SB and Edwards MJ The Effect of Temperature and Pulsed Ultrasound on the Development of Rat Embryos in Culture, Teratology 42: 285-293 SCHEDULE OF WORK TO BE PERFORMED: 1. DNA samples are already available for marker analysis. Selection of markers to replace poorly informative markers is anticipated at the start of the grant. Preliminary analysis of markers using pooled samples for affecteds, carriers and clears anticipated within the first three months of grant. 2. Acquisition of marker allele data for linkage families is anticipated to be complete at the end of the ninth month of the grant. Completed marker data will be forwarded to Dr. Famula for analysis. 3. Completed analysis for linkage by end of grant. Publication at end. BUDGET: The budget of $70 000 will support the salary of a technician at University College Dublin to assist in sample preparation and preparation of gels for microsatellite analysis. The Veterinary Genetics Laboratory at UCDavis has indicated that they will run samples at $1/marker, and that the multiplex will have at least 200 markers at the time of this study. Personnel: University College Dublin Technical Support Subtotal Salaries Materials and Supplies: University College Dublin Reagents Consumables Subtotal Materials and Supplies Subcontract for Multiplex markers University of California, Davis 200 markers/dog for 150 dogs $1/marker Subtotal Subcontract Multiplex Markers $ 15, 000 ________ $ 15, 000 $ 15, 000 $ 10, 000 ________ $ 25, 000 $ 30, 000 ________ $ 30, 000 TOTAL ________ $ 70, 000 Amount Requested from Canine Health Foundation: ________ $ 70, 000