Nene-Ozone
advertisement
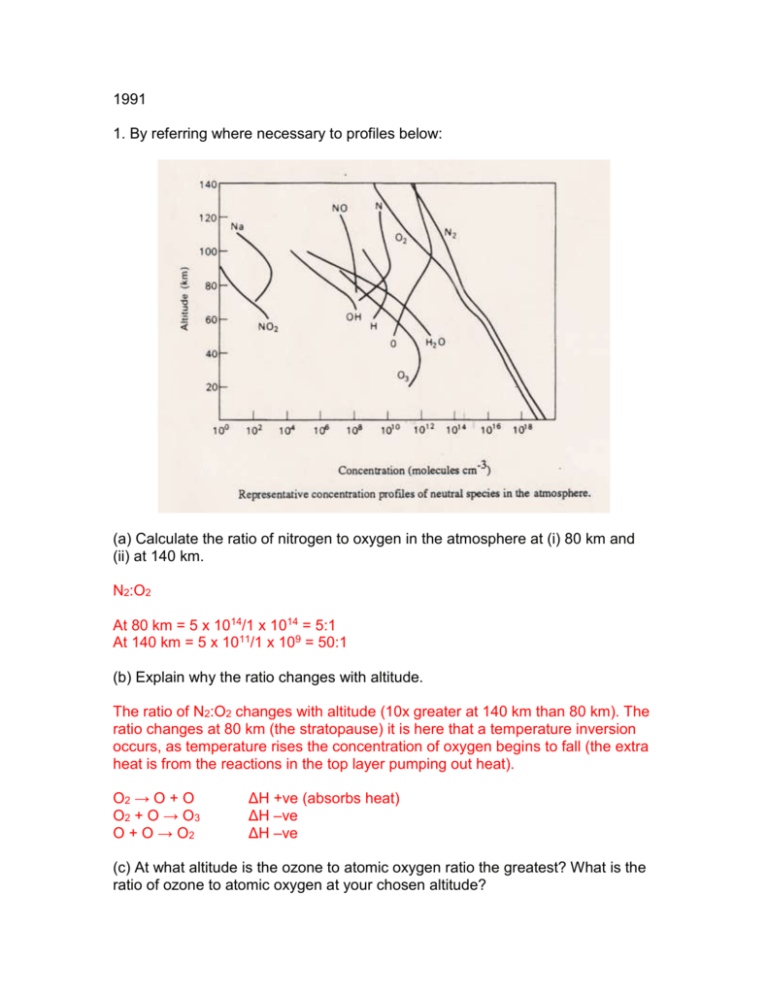
1991 1. By referring where necessary to profiles below: (a) Calculate the ratio of nitrogen to oxygen in the atmosphere at (i) 80 km and (ii) at 140 km. N2:O2 At 80 km = 5 x 1014/1 x 1014 = 5:1 At 140 km = 5 x 1011/1 x 109 = 50:1 (b) Explain why the ratio changes with altitude. The ratio of N2:O2 changes with altitude (10x greater at 140 km than 80 km). The ratio changes at 80 km (the stratopause) it is here that a temperature inversion occurs, as temperature rises the concentration of oxygen begins to fall (the extra heat is from the reactions in the top layer pumping out heat). O2 → O + O O2 + O → O 3 O + O → O2 ΔH +ve (absorbs heat) ΔH –ve ΔH –ve (c) At what altitude is the ozone to atomic oxygen ratio the greatest? What is the ratio of ozone to atomic oxygen at your chosen altitude? 50 km O3:O = 1 x 1012/1 x 1010 = 100:1 (d) Describe clearly how the temperature of the atmosphere varies between the altitude of 10 km and 100 km. Explain the reason for this trend. Near the surface the temperature decreases with increasing altitude, this region up to around 10 km is turbulent and well mixed and is referred to as the troposphere. The temperature passes through a minimum at the tropopause above which it rises with increasing altitude up to a maximum at the stratopause this is due to the 3 reactions in the top layer pumping out heat. Temperature then decreases at the mesopause (60 km) because of there being much less oxygen to react. At 100 km another inversion occurs as the temperature increases this is the thermosphere where atoms are losing electrons to form ions (exothermic process) and a lot of chemical reactions produce heat, also kinetic energy from impacts. (e) What is the major difference between the homosphere and the heterosphere? What is the altitude that marks the separation between homosphere and heterosphere? The homosphere is a region below 85 km, constant chemical composition is maintained by slow diffusion which is governed by wind turbulence. The heterosphere is the region above 85 km, changing diffusion is of great importance. (f) Explain why, although light of wavelengths less than 200 nm contribute to incident solar radiation, at sea-level the short-wavelength cut off is about 320 nm. Some solar radiation is absorbed, scattered and reflected by atmospheric gases and clouds. 2. (a) Discuss the relationship between the measured and predicted concentrations of nitric oxide (NO) in the atmosphere, up to an altitude of 110 km. (b) Briefly describe how the observed concentrations are measured. Aircraft at 30-40,000 feet, balloons, rockets and satellites. 1991 4 (a) Fully describe the Chapman mechanism for the production of stratospheric ozone. (b) Discuss how NOx species effect the steady state concentration of zone. (c) Outline the mechanism for the photochemical production of tropospheric ozone and comment on one environmental problem for this gas. 1992 2. (a) Discuss the relationship between the measured and predicted concentration of nitric oxide in the atmosphere, up to an altitude of 100 km. (b) Briefly describe how the observed concentrations were measured. (c) What is the major difference between the homosphere and the heterosphere? 3. (+1990) (a) Describe the classification of the earth’s atmosphere into regions, based on temperature. In which region is the so called ‘ozone layer’? (b) Explain the occurrence and function of ozone in this region. (c) Discuss how anthropogenic activities are thought to be capable of influencing the levels of ozone in this region. 4. (a) Explain why CFC-11 (CFCl3) is resistant to hydrolysis in the troposphere. (b) Suggest why lipids such as oleic acid (C18H34O2) react with ozone whereas polymers such as Teflon -(CF2-CF2)n- would not.