Assignment4 Practice - Dutton e
advertisement

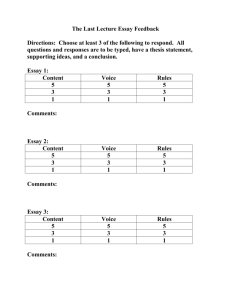
Assignment 4, Practice. Fall 2008. A Materials’ Essay; Part 2 1. Introduction to Practice Assignment 4. In this practice for assignment 4, you will find a rogue essay on “The Structure of Oxygen”, together with my analysis of the essay, presented as feedback to the 25 grading questions. You will also find an essay on “The Structure of Oxygen”, which is written to the best of my ability, also with feedback. If you study the two essays, together with my feedback, you should be able to comprehend my ideas on good scientific writing; you should also be able to obtain a good grade on assignment 4! 2. Specification for the Structure Essay1. The essay should provide the reader with an introduction to the structure of copper. The text, excluding figure captions, references, etc., should be at least 200 words in length and should be typed in Times 12-point. The essay should be drawn from at least two independent sources, which must be cited in the text and also listed after the text. The text must include at least one graphic (photograph, schematic drawing etc.). The figure should be accompanied by a figure caption and the source of the figure must be acknowledged. Other details are made explicit in Section 3. 3. The Grading Questions A. A. Use of English (10) 1. Does the first sentence of the essay accurately introduce and/or reflect the subject matter of the essay? Yes (2) No (0) 2. Is the text grammatically correct, properly punctuated and free of spelling/typographical errors?2 Yes (0) No (-2) 3. Is the essay free of inconsequential verbiage? Yes (2) No (0) 4. Is the essay free from colloquialisms3: words or phrases that are part of the vernacular? 1 My "system" is based on "Calibrated Peer ReviewTM: A Writing and Critical Thinking Instructional Tool". (Workshop Handbook). K. M. Brand and A. A. Russell. Copywright 2001, Regents of the University of California. 2 What is grammar? What is punctuation? The following definitions, and those in similar footnotes, have been abstracted from Webster's New World Dictionary. 2 nd College Edition. David B. Guralnik, Editor in Chief. Simon and Shuster. New York, NY. (1982) Grammar: "…that part of the study of language, which deals with the forms and structures of words (morphology), with their customary arrangement in phrases and sentences (syntax)… a body of rules for speaking and writing a given language…” Punctuation: "…the act, practice, or system of using standardized marks in writing and printing to separate sentences or sentence elements or to make the meaning clearer". 3 Colloquial: “1. having to do with or like conversation; conversational” Yes (2) No (0) 5. Is the essay free from hyperbole4, or exaggeration? Yes (2) No (0) 6. Is each sentence, free of non-sequiturs5, e.g., does the second part of a sentence bear a relationship to the first part? Yes (2) No (0) B. Content (10) 7. Does the essay describe the electronic structure of the material? Yes (2) No (0) 8. Is the nuclear structure discussed, e.g., are isotopes identified, and/or listed? Yes (2) No (0) 9. Does the essay define/describe the nature of the chemical bonds in the material? Yes (2) No (0) 10. Does the essay state whether the material is crystalline or amorphous? Yes (2) No (0) 11. If the material is crystalline, is the crystal structure of the material clearly stated? If the material is amorphous, is the term “amorphous” clearly defined? Yes (2) No (0) C. Organization (4) 12. Is the content presented in a logical fashion, Does one sentence flow naturally from the one preceding? Yes (4) No (0) D. Scientific Accuracy and Attribution (6) 13. Is the essay free of scientific errors?6 Yes (2) No (-2) 4 Hyperbole: “exaggeration…” Non sequitur: “1. Logic a conclusion or inference which does not follow from the premises…2. a remark having no bearing on what has just been said” 6 You might reasonably object, on the grounds that you are not qualified to comment on scientific accuracy. Whereas this might be true in an absolute sense, you are able to check that the results quoted in the source materials are accurately reported in the essays. For the present purposes, you may assume that the results quoted in the sources are correct: this is not always a good assumption. 5 14. Are the source materials correctly, and adequately cited in the text? Yes (2) No (-4) 15. Are the source materials listed appropriately, as either footnotes, or at the end of the essay? Yes (2) No (-4) 16. Is the text of the essay clearly the work of the author, i.e., is it free of plagiarism7? Yes (0) No (-4) E. Use of Graphics and Tabular Materials (4) 17. If graphics (figures) and/or tabular materials are used, are they referred to adequately in the text? If neither graphics nor tabular materials are used, score a zero. The maximum score is four points, irrespective of the number of illustrations/tables used. If graphics and/or tabular materials are used, but are not referred to in the text, score a -2. Yes (4) No (-2) 18. If graphics are used, are they accompanied by figure captions? Is the source of the graphics acknowledged? If graphics are not used, score a zero. Yes (0) No (-4) 19. If graphics are used, are they numbered consecutively? If graphics are not used, score a zero. Yes (0) No (-2) 20. If tables are used, does a table number accompany them? If tables are not used, score a zero. Yes (0) No (-2) 21. If tables are used, are they accompanied by a table heading? Is/are the source(s) of the tabular material acknowledged? If tables are not used, score a zero. Yes (0) No (-4) 22. If tables are used, are the listed parameters explained, and/or defined as footnotes to the table, or in the text? If tables are not used, score a zero. Yes (0) No (-4) F. Formatting (6) 23. Is the essay formatted for ease in reading and comprehension? Yes (2) No (0) 7 Plagiarize: “ to take (ideas, writings, etc,) from (another) and pass them off as one’s own” 24. Is the text of the required length (200 words minimum)?8 Yes (2) No (-2) 25. Is the text single-spaced, Times, 12 point? Yes (2) No (0) MAXIMUM SCORE G. Overall Rank 26. The Overall Ranking of the essay is given by the sum of questions 1-26 30+ 20+ 10+ Less than 10 8 Excellent Good Poor Remedial Action Needed Does this mean that you (the student) must determine, or estimate the word count? Yes! 40 POINTS The Structure of Oxygen (Rogue Essay). There is no need to make oxygen in the laboratory because it is readily available commercially or through air liquefaction plants. In fact, oxygen is the most abundant terrestrial element. It has an atomic number of 8, hence 8 protons and 8 neutrons. In a neutral atom, it has eight electrons orbiting the nucleus as shown in the Figure below, which is a schematic representation of the shell structure of oxygen – not what the atom of oxygen “looks like”. Oxygen has three naturally occurring isotopes. Oxygen is a gas with a monoclinic crystal structure as shown below. Isotope Atomic mass (ma/u) 16 O 17 O 18 O 15.99491463 (5) 16.9991312 (4) 17.9991603 Natural abundance (atom %) 99.757 (16) 0.038 (1) 0.205 Nuclear spin (I) 0 Magnetic moment (m/mN) 0 5/2 -1.89380 0 0 (9) (14) Space group: C12/m1(Space group number :12) Structure: monoclinic Cell a/pm b/pm c/pm Parameters 540.3 342.9 508.6 90.00 132.53 90.00 Oxygen forms chemical bonds with virtually all other elements. The most famous of the oxides is dihydrogen oxide (water) and compounds of carbon and oxygen such as carbon dioxide (CO2) alcohols (R-OH), and aldehydes (R-CHO). References. [1] WebElementsTM, the periodic tale on the WWW. Mark Winter: The University of Sheffield and WebElement Ltd., UK © Mark Winter, 1993-2008. .Accessed 07/03/08 http://www.webelements.com/webelements/elements/text/O/key.html [2] “Oxygen”. From Wikipedia, the free encyclopedia. Licensed to the public under the GNU Free Documentation License. Accessed 06/16/08 http://en.wikipedia.org/wiki/Oxygen Feedback. Essay # 2. Number Answer Feedback 1. No The first sentence is irrelevant to an essay that is describing structure. It might be relevant if the essay were discussing processing. 2. Yes The style is poor but the mechanics appear to be OK 3. No The second sentence begins with: “In fact…” The phrase “In fact” is meaningless: either oxygen is, or is not the most abundant terrestrial element (it is). In sentence 4, the phrase “… not what the atom of oxygen “looks like”” is unnecessary; earlier in the same sentence, the figure is described as a “… schematic representation”. 4. Yes (?) A borderline yes! The phrase “In fact…” is colloquial, and should not be used in formal writing. 5. Yes 6. No The sentence “It has an atomic number of eight, hence 8 protons and 8 neutrons” is illogical. The atomic number cannot be used to determine the number of neutrons. The sentence “Oxygen is a gas with a monoclinic crystal structure…” is also a classic nonsequitur: a gas cannot be crystalline. 7. Yes The description is meager and barely sufficient. 8. Yes The last sentence of the first paragraph mentions isotopes, and a list is provided in the table, which is not referred to in the text 9. No 10. No Nowhere is it explicitly stated that oxygen is crystalline. 11. No It is stated that “Oxygen is a gas with a monoclinic crystal structure…” which is a non-sequitur. A list of meaningless parameters, such as “Space group” is also provided, but not mentioned in the text. 12. No The essay begins with a statement about processing, gives some garbled structural information and finishes by listing several oxygen-bearing compounds. 13. Yes Something must be right! 14. No The source materials are not cited at all. Hence, the essay is plagiaristic. The last paragraph is virtually a direct quote from ref [2]. The figures and tables are copied directly from ref [1] 15. Yes 16. No The author is guilty of plagiarism, for the reasons enumerated in the answer to question 14. 17. No The figures are only alluded to using the phrase “as shown below”. They are not numbered. The tabular material is not referred to at all, making the information worthless. 18. No There are neither figure numbers, nor captions (-2). There is no acknowledgment regarding the source of the figures (-2) 19. No Score 0 0 0 2 2 0 2 2 0 0 0 0 2 -4 2 -4 -2 -4 -2 20 21 No No 22 No 23 No 24. 25 No Yes There are neither table numbers, nor table headings (-2). There is no acknowledgment regarding the source of the tabular materials (-2) I wonder if the author knows what “nuclear spin” and “magnetic moment” signify? Figures, tables and text are all mixed together, making it difficult to “navigate” through the essay. By my count, the word total (text only) is 134 QUANTITATIVE SCORE -2 -4 -4 0 -2 2 -14 The Structure of Oxygen. Oxygen is in period 2, group 16 of the periodic table [1]: in its standard state at 25˚C, it is a colorless, odorless gas. Oxygen has an atomic number of eight, which means that its nucleus contains eight protons, and for a neutral atom, there are eight orbiting electrons. The ground state (lowest energy) electronic structure for oxygen [1] is: [He].2s2.2p4 which is equivalent to a shell structure of 2.6 [1]. Its atomic weight, which is approximately equal to the number of protons, plus the average number of neutrons in the nucleus, is 15.9994 [1]. Oxygen’s three stable isotopes are 16 O(99.757%), 17O(0.0380%) and 18O(0.205%) [1]. It is not possible to define a unique atomic radius to oxygen, or indeed any element, because this radius varies with the environment of the atom. Perhaps the most meaningful are the covalent radius (0.73nm) and van der Waals radius (1.652nm) [1], which yield the interatomic distance within a molecule (covalent), and the somewhat larger intermolecular distance (van der Waals). The covalent and van der Waals radii are indicated on Figure 1a. Figure 1. Schematic diagrams of the crystal structure of solid (crystalline) oxygen. In both cases, the orientation of the unit cell is shown in the lower left-hand corner (adapted from ref. [1]). a). Ball and stick representation. a). Hard-sphere model. In a), the covalent radius is shown to correspond with (half) the interatomic spacing, within oxygen molecules. The van der Waals radius is characterized by (half) the intermolecular spacing. Although a gas at room temperature, if sufficiently cooled, oxygen will liquefy and finally solidify (boiling and melting points of –182.9˚C and –218.3˚C respectively [1]). In common with most solids, the solid form of oxygen is crystalline, where ref [2] defines a crystal as: “… a crystal is a solid in which the constituent atoms, molecules, or ions are packed in a regularly ordered, repeating pattern…” Figures 1a,b are schematic drawings of the crystal structure of oxygen, which is monoclinic [1], with unit cell parameters, a=0.5403nm, b= 0.3429nm and c=0.5086nm. As noted in [3], a crystal structure can be described by: “…a unique arrangement of atoms in a crystal …. a motif, a set of atoms arranged in a particular way…” The orientation of the unit cell is indicated in the lower left hand corner of Figures 1a,b. Note, however, that the unit cell is not of the correct size References. [1] WebElementsTM, the periodic tale on the WWW. Mark Winter: The University of Sheffield and WebElement Ltd., UK © Mark Winter, 1993-2003. http://www.webelements.com/webelements/elements/text/O/key.html [2] “Crystal”. From Wikipedia, the free encyclopedia. Licensed to the public under the GNU Free Documentation License. Page last modified Aug.11, 2005. http://en.wikipedia.org/wiki/Crystal [3] “Crystal Structure”. From Wikipedia, the free encyclopedia. Licensed to the public under the GNU Free Documentation License. Page last modified Aug.11, 2005. http://en.wikipedia.org/wiki/Crystal_structure Feedback. Essay #1. Number Answer Feedback 1. Yes The first sentence introduces some structural aspects of oxygen: it serves as a springboard for the remainder of the essay. 2. Yes 3. Yes 4. Yes 5. Yes 6. Yes 7. Yes Both the “ground state” and “shell structure” of oxygen’s electronic structure are presented (end of first paragraph). 8. Yes The number of protons is presented in paragraph 1, and the stable isotopes of oxygen are listed in paragraph 2. 9. No 10. Yes See second sentence of fourth paragraph “… the solid form of oxygen is crystalline…” 11. Yes In the quote following the fourth paragraph, a crystal is defined, and the fifth paragraph describes the crystal structure of solid oxygen, which is also shown in the figures, which are referred to in the text. 12. Yes The essay progresses from a description of electronic structure, to a listing of isotopes, to a discussion of atomic radius, and finally to crystal structure. 13. Yes 14. Yes/No The citation practice is generally OK. However, no citation accompanies the second paragraph: the data are from ref. [1] Note that I am using a numerical system with the reference numbers being located at the end of the passage to which it refers. Also note also that the period (full-stop) is located after the reference, and the references are numbered sequentially. 15. Yes In keeping with the numerical system, the references are listed sequentially following the text. 16. Yes 17. Yes The fifth paragraph refers to, and describes the figures. 18. Yes Note that the first part of the caption is not a full sentence: it is a description. The rules for figure captions are somewhat different from normal text: figure captions should be descriptive. 19. Yes In this instance, there is one figure consisting of two parts, a) and b), which are referred to in the text, and labeled on the figures. 20 NA 21. NA 22 NA 23 Yes Paragraphs are delineated by leaving spaces, and the figure caption is distinguished from the text by a change in font Score 2 0 2 2 2 2 2 2 0 2 2 4 2 0 2 0 4 0 0 0 0 0 2 24. 25. Yes Yes Word count, text only: 321 QUANTITATIVE SCORE 2 2 34