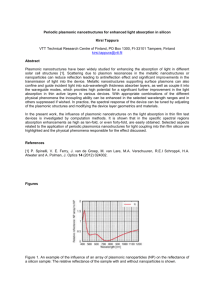
3. Safety assessment of nanostructures in food
advertisement

Nanotechnology in food production: a potential risk or a risky potential? On the potential of emulsification techniques to generate nanostructures for the use in food and the risk and safety considerations Aidan Noorman Utrecht University December 2008 Supervisors: Raymond Pieters, PhD Institute for Risk Assessment Sciences Utrecht University, Utrecht, the Netherlands Nico van Belzen, PhD International Life Science Institute, Brussels, Belgium Nanotechnology in food production: a potential risk or a risky potential? │ i Nanotechnology in food production: A potential risk or a risky potential? On the potential of emulsification techniques to generate nanostructures for the use in food and the risk and safety considerations Master thesis by: Aidan Noorman MSc program Toxicology and Environmental Health Faculty of Biomedical Sciences, Utrecht University, Utrecht, the Netherlands e-mail: G.A.Noorman@students.uu.nl Nanotechnology in food production: a potential risk or a risky potential? │ ii Abstract The purpose of this report was to investigate the safety assessment of emulsification techniques and the variety of emulsion-based nanostructures (EBNS) that can be obtained and subsequently used in the food industry. Environmental issues will not be addressed. The number of nanotechnology applications is increasing and it is expected that the food industry will be the newest field in which nanotechnology will be applied. A conservative size definition of 500 nm was chosen for risk assessment purposes. Cells are capable of taking up nanostructures of up to 500 nm in size and nanostructures can be engineered with certain properties that could mimic effects of smaller sized nanostructures. Devices to produce emulsions and EBNS are already used in the food industry (homogenisers) while other methods are still being developed which are more efficient (low-energy methods). A large variety of nanostructures can be obtained with emulsification techniques such as simple emulsions, lipid nanostructures, solid nanostructures etc. Very little is known about the effects of nanostructures on the gastrointestinal tract. Nanostructures in the body mostly accumulate in the liver and kidneys where the effects are the most pronounced. Surface properties are very important as they can determine the fate, function and possible risks of nanostructures. More research is needed in which other non-metallic and non-carbon-based nanostructures are (orally) tested. Recommendations for a food nanostructure-specific risk assessment include the goal of the nanostructure (intended or unintended exposure), consideration of physicochemical properties relevant to food nanostructures (e.g. solubility), the history of safe use and a minimum amount of testing to ensure safety. A risk assessment paradigm is proposed which incorporates these recommendations. Nanotechnology in food production: a potential risk or a risky potential? │ iii Table of contents 1. INTRODUCTION ----------------------------------------------------------------------- 1 1.1 Size definitions ........................................................................................... 2 2. EMULSIONS AND APPLICATIONS----------------------------------------------- 4 2.1 Nano fabrication ........................................................................................ 4 2.2 Emulsions as template............................................................................... 4 2.2.1 High-energy methods ................................................................................ 5 2.2.2 Low-energy methods ................................................................................. 6 2.3 Applications of emulsion based nanostructures ..................................... 7 2.3.1 Traditional emulsions ............................................................................... 7 2.3.2 Nano-emulsions as carriers: nanocapsules .............................................. 8 2.3.3 Nanoliposomes.......................................................................................... 8 2.3.4 Solid nanoparticles ................................................................................... 9 2.3.5 Colloidosomes........................................................................................... 9 2.4 Developments and trends........................................................................ 10 3. SAFETY ASSESSMENT OF NANOSTRUCTURES IN FOOD ------------- 11 3.1 Potential risks .......................................................................................... 11 3.1.1 Importance of preparation methods........................................................ 11 3.1.2 Nanostructures in the gastrointestinal tract ........................................... 11 3.1.3 Biodistribution ........................................................................................ 12 3.1.4 Effects of surface properties ................................................................... 12 3.1.5 Effects on the immune system ................................................................. 14 3.1.3 Other routes of exposure ........................................................................ 15 3.2 A food-specific risk assessment .............................................................. 15 4. CONCLUSIONS ----------------------------------------------------------------------- 21 5. REFERENCES ------------------------------------------------------------------------- 22 Introduction │ 1 1. Introduction Nanotechnology and nanoscience deal with the development, application and production of materials with sizes less than 100 nm. Materials with such dimensions posses unique properties that are not exhibited by larger structures of the same material [Oberdörster et al., 2005b]. Research and development into nanotechnology is an increasing business. In the US, the budget of the National Nanotechnology Initiative (NNI) has increased from $464 million in 2001 to $1.4 billion in 2007 and is estimated to increase to $3 billion in 2009 [NNI, 2008]. In Europe, funding is mostly provided by national governments and totals around €1 billion. Only a very small percentage of the funding is invested in safety assessment, life cycle analysis, monitoring or tracking systems [Powell et al., 2008]. For now, many scientists and companies are more interested in the technological and economical benefits that nanotechnology may hold for the future [Renn and Roco, 2006]. Development of nanotechnology occurs in many different fields and disciplines such as electronics, cosmetics, pharmaceutics, agriculture etc. One of the newer fields where nanotechnology is making an entrance is food. It is known that a large part of the general population is already exposed to nanotechnology, via cosmetics [Thomas et al., 2006] and now an even larger part may be exposed through the food chain. In February 2008 an online survey by the Woodrow Wilson International Centre for Scholars (WWICS) identified over 600 products that contain some form of nanomaterial [WWICS, 2008]. Of the 600 products 69 where found to be related with food, this includes mostly supplements, storage devices and kitchen equipment but this is expected to be more, since this is only an online survey and no supermarket products have been tested. There are four major areas in food production to which nanotechnology can be applied: food processing, packaging and storage, food safety and functional foods (food with additional heath-promoting functions). Food packaging and storage is so far the most promising area for nanotechnology and some of the innovations include oxygen barriers [Erlat et al., 2001; Sorrentino et al., 2007], antimicrobial films [Rhim et al., 2006] and thermal buffering techniques for perishable foods [Johnston et al., 2008]. Food safety mostly includes detection and elimination of foodborne pathogens and toxins [Branen et al., 2007; Yang et al., 2008]. Nanotechnology in food processing and functional foods is raising both interests and concerns because this involves direct application into the food itself. The use of nanostructures in food can affect the appearance, smell and taste of a product [Graveland-Bikker and de Kruif, 2006; Rodríguez Patino et al., 2008] or reduce costs and/or environmental burden [Vincze and Vatai, 2004]. Some of the techniques and principles to generate nanostructures are actually not new. An example is the generation of nanofibers. One way to make nanofibers is through a process called electrospinning. The technology for this was already invented in the early 1900s for polymer filaments, and in the 1990s the technique was refined to produce nanofibers [Li and Xia, 2004]. With these fibres it was possible to produce nanofilters [Ramakrishna et al., 2006] that eventually spawned the technology for membrane emulsification [Charcosset et al., 2004; Kampers, 2007]. This report will focus on nanostructures that can be obtained through emulsification. Emulsification is the mixing of two immiscible fluids and is a process that has been used for a very long time in food processing. Emulsification is mainly used in dairy products and beverages but nowadays it is also possible to create nanostructures with it. The capabilities of older techniques and principles to generate nano-emulsions and emulsion-based nanostructures (EBNS) will be investigated for their use in food. As EBNS can have various compositions and characteristics [Daufin et al., 2001] different risks have been associated with nanostructures. Therefore, the emphasis of this report will be on the safety assessment of EBNS. The first half of this report will Introduction │ 2 provide a short elaboration of the methods to generate nanostructures and their possible impact on food. The second half of this report is on the risk assessment aspect for nanotechnology in food. A risk assessment was recently developed by the Scientific Committee on Emerging and Newly-Identified Health Risks (SCENIHR) that applies to nanotechnology in general. Critical views and recommendations will be provided based on possibilities of EBNS in order to make the tiered risk assessment more specific to the food industry. 1.1 Size definitions In the scientific community there is a general tendency to define structures with at least one dimension that measures less than 100 nm as a nanostructure. For toxicological and safety assessment purposes this definition may not be sufficient. Cells are capable of taking up particles of 300 nm, this include modes not involving receptors. Lately, a process has been described that can even take up 500 nm particles [Garnett and Kallinteri, 2006]. Also, the ‘engineered’ aspect of >100 nm engineered nanostructures can give similar capabilities as <100 nm nanostructures. So, in order to set a strict definition of the term ‘nano’ that can be used throughout the whole scientific community, a consensus will have to be reached between toxicologists and scientists dealing with structural engineering of nanostructures. Making agreements on a general definition of the term nano is not easy, even in a specific field. There are several reasons for this. One is the fact that the size definition of the word nano differs between scientists. Emulsions are categorised based on their droplet sizes. From the commonly used definition of nano, we would expect that ‘nano-emulsions’ are <100 nm in their droplet sizes. Several scientists do indeed use this definition [Meleson et al., 2004] but there are also other scientists that employ a different size (range) such as 20-200 nm [Solans et al., 2005; Tadros et al., 2004] or even 100-1000 nm [Windhab et al., 2005]. In the field of emulsion research the label ‘nano-emulsion’ where we assume approaches the general definition of ‘nano’ (<100 nm), there are multiple synonyms such as mini-emulsion, submicron emulsions, ultrafine emulsion and microemulsions [Solans et al., 2005]. Lately, the term nano-emulsion has been gaining popularity due to the ‘nano’-hype in society and the scientific community. Thus, not only is the very definition of the term nano being debated, also the terminology has not been standardised. In light of all these aspects it will be hard to define a ‘true’ nano-emulsion. A search in the literature for the term ‘nano-emulsion’ and several of its synonyms yielded nanostructures with average sizes that were above the 100 nm. Considering this result, the uptake capacity of cells and the fact that nanostructures can be engineered to have certain properties, it would seem appropriate to also include the structures that are 100 nm and larger in size. The next step would be to determine a proper upper limit. From a toxicological and risk assessment point of view, uptake sizes of 300 nm or even 500 nm are an important aspect to consider. Furthermore, a distinction is often made between smaller (<500 nm) and larger (>500 nm) micron particles [Oberdörster et al., 2005b; VegaVilla et al., 2008] while others do consider 500 nm as the upper limit [Anton et al., 2008]. For now, a conservative upper limit of 500 nm will be used for food risk assessment purposes. The idea of implementing nanotechnology in food is new, and so, we can expect that the number of literature sources with respect to engineered nanostructures in food to be limited. In this report we will describe EBNS less than 500 nm in size that may be suitable for food applications. For the assessments of potential dangers both in vitro and in vivo studies of EBNS (<500 nm) are used. However, other non-EBNS studies are used in order to describe effects of various properties. Also, pharmaceutical studies with nano-emulsion formulations will be considered. Pharmaceutical research on nanostructures has been going on longer than in food research, including Introduction │ 3 oral formulations. For now, pharmaceutical studies may be the biggest source of information on engineered nanostructures in the human body. Emulsions and applications │ 4 2. Emulsions and applications Emulsion is the mixture of two immiscible liquids, where one liquid is dispersed (dispersed phase, e.g. in droplets) in the other (continuous phase). A nano-emulsion is an emulsion with droplet sizes that range up to several hundred nm. For reasons discussed in the introduction the upper limit of 500 nm will be used. Nanoemulsions can present characteristics and properties which depend on the composition and preparation method. In this chapter a general principle of nanostructure generation will be explained. Next, several methods to generate nanostructures will be described. An elaboration will be given on the kind of nano-emulsions and EBNS that can be obtained by these methods. The chapter will be concluded with a critical view on what aspects of nanostructure generation and developments can be considered new. 2.1 Nano fabrication In general, the production of nanostructures can be accomplished in two ways: via a bottom-up approach or a top-down approach. The top-down approach is the down-sizing of macrostructures. This is usually accomplished by applying a force in the form of shear and impact. Devices capable of this are milling devices and homogenisers. Advantage of this method is that the amount of force, and thus the size can be easily controlled. Top-down approaches are currently the most widely used methods in large scale industries [Sanguansri and Augustin, 2006]. The bottom-up approach deals with the manipulation and/or controlled (self-)assembly of individual molecules to create nanostructures. This method of production is not new in nature: in the body proteins and enzymes are formed by amino acids which themselves are controlled by ribosomes. Examples of man-made assembled nanostructures are the various carbon nanostructures such as nanotubes and fullerenes. This process is more tedious and more susceptible to failure than the top-down approach due to the heavy dependence on the physicochemical properties and the environment in which the reactions take place. The advantage, however, is that there is a far greater chemical variety [Seeman and Belcher, 2002]. It is expected that the use of this approach will further increase as nanotechnology matures [Sanguansri and Augustin, 2006]. 2.2 Emulsions as template The creation of nano-sized structures can be accomplished in many different ways. One of them is by basing it on an emulsion-template. Emulsions are a versatile system that can be used for many different products, including standard food emulsions, formation of nanospheres or powders, nanobeads for processing or flavour enhancement, etc. [Vladisavljević and Williams, 2005]. Characteristics of an emulsion depend for a great deal on the size of the droplets. For example emulsions with large droplets (>500 nm) have a milky appearance whereas emulsions with small droplets (<200 nm) are clear (see fig. 1). There are many different kinds of emulsions and many different ways to produce them. Here, an explanation is given of some of the methods that are capable of producing nano-emulsions. Several of Figure 1 Example of the difference between a 35 nm nano-emulsion (A) and a normal 1000 nm emulsion (B). Modified from [Solans et al., 2005]. these methods are already in use by the food industry but explanations are also given of some new methods that Emulsions and applications │ 5 can serve as a potential alternative to the current ones. Emulsions used in the explanations here are the standard emulsion, consisting of water and oil. 2.2.1 High-energy methods The methods of producing nano-emulsions can be roughly divided into two categories: high-energy and lowenergy methods. High-energy methods are now being used in the industry. The name is derived from the fact that the energy that is applied to break up the droplets is very high but this method may not always be efficient [Tadros et al., 2004]. Two high-energy methods will be explained that are currently used in the food industry. 2.2.1.1 High pressure homogenization High pressure homogenisation is a technique that employs shear stresses to break up droplets. The fluid with (preformed) emulsions is forced through microchannels where extreme shear stresses are generated due to the sudden restriction of the flow under high pressure combined with acceleration, compression, turbulence, impact and eventually rapid pressure drop. Figure 2 shows several different types of high pressure homogenisation systems which all work with the same principles [Schultz et al., 2004]. Microfluidizer systems are the most effective in producing nano-emulsions [Meleson et al., 2004; Pinnamaneni et al., 2003]. Figure 2 Different types of high pressure homogenisation systems. Modified from [Schultz et al., 2004]. 2.2.1.3 Ultrasound Emulsification through ultrasound is also an effective way of producing nano-emulsions. Ultrasonication generates high and low pressure waves which lead to the formation of vacuum bubbles in both fluids (i.e. water and oil), which is also known as cavitation. These bubbles implode, causing extreme conditions with temperatures of up to 5000 K and pressures of over 1000 atmosphere (≈101 MPa) [Patist and Bates, 2008]. The implosions produce shockwaves, which in turn result in levels of highly localised turbulence. The heat, pressure and turbulence are effective means of breaking up and dispersing large droplets in a liquid medium. Emulsions and applications │ 6 Ultrasonication is a technology that has been used on a laboratory scale for many years. But recently (<5 years), ultrasound devices have been developed for industrial uses. 2.2.2 Low-energy methods Beside the more traditional high-energy methods for nano-emulsions, there are also methods that do not require large amounts of applied energy, the so-called low energy methods. Three of such methods have been described in the literature and will be briefly explained here as potential alternative to the high energy methods. Detailed descriptions of the processes governing the reactions are beyond the scope of this report and only basic explanations will be given. 2.2.2.1 The solvent displacement method Solvent displacement utilises the physicochemical properties of components to form nano-emulsions. Solvent displacement consists of three components. First, a solute (e.g. oil) is dissolved in a solvent (e.g. ethanol) and afterwards a third component (e.g. water) is added. The third component is miscible with the solvent but not with the solute. During the addition of the third component small droplets are formed containing the solute/solvent mix. Spontaneous emulsification can occur when diffusion of the third component into the solute/solvent droplet causes the solvent to be displaced from the droplet, leaving behind small nuclei of the solute. Since the third component is immiscible with the solute nano-sized emulsions are rapidly formed. This method of producing emulsions was termed the ‘ouzo effect’ because the original authors compared the processes with the addition of water to ouzo, a common alcoholic beverage in Greece [Vitale and Katz, 2003]. The advantage of solvent displacement is that it does not require surfactants/emulsifiers. Also, the whole process occurs rapidly and over the entire volume which makes it interesting for industrial applications. 2.2.2.2 Phase inversion temperature Phase inversion temperature (PIT) utilises the physicochemical properties of the surfactant to obtain nanoemulsions or EBNS. It is based on the interruption of the transition of an oil-in-water (O/W) phase into a waterin-oil (W/O) phase. For instance, when the temperature rises, the surfactant is modified in such a way that it exhibits equal affinity for both fluids (e.g. water and oil). This is when the O/W phase starts to go over into a W/O phase. During the transition between phases nano-emulsions are formed due to low interfacial tension. However, coalescence is extremely fast which will eventually result in a coarse emulsion [Solans et al., 2005]. To prevent coalescence, the temperature is suddenly and rapidly lowered in order to preserve the current state (i.e. the transition state where nano-emulsions have formed). Due to the temperature change, the surfactant exhibits normal affinity for one of the fluids [Anton et al., 2008]. 2.2.2.3 Membrane emulsion Membrane emulsification does not involve physicochemical properties of the components, but instead uses a membrane with nanopores. A liquid-to-be-dispersed is slowly forced through the membrane. Mechanical agitation, usually in the form of a flow, is used to detach the newly formed droplets at the membrane pores. Membrane emulsification requires less energy than high pressure homogenisation and ultrasound, making it an interesting option for large scale use. A disadvantage is the low flux of the liquid-to-be-dispersed through the membrane, which means a longer production time [Charcosset et al., 2004; Vladisavljević and Williams, 2005]. Emulsions and applications │ 7 2.3 Applications of emulsion based nanostructures The methods described above are capable of producing a wide variety of nanostructures. Aside from the usual emulsions, various other nanostructures can be obtained. Figure 3 shows several nanostructures that can be obtained based on an emulsion template. In this section an explanation is given of the general features of the various EBNS and how they can be obtained. Figure 3 Different types of emulsions and EBNS obtainable through various methods of emulsification. Modified from [Vladisavljević and Williams, 2005]. 2.3.1 Traditional emulsions The traditional O/W and W/O emulsions are the most common product of emulsification techniques. Advantages of nano-sized emulsions are improved stability, shelf life, digestion, taste, appearance (e.g. fig. 1) and flow properties [Sanguansri and Augustin, 2006]. Consumer products that directly benefit from nano-emulsions are baby foods, purees and beverages (through high-energy methods). W/O emulsions have been successfully used in spreads and margarines [Joscelyne and Trägårdh, 1999]. More complex emulsions are the double emulsions, water-in-oil-in-water (W/O/W) and oil-in-water-in-oil (O/W/O, fig. 3). These emulsions are used for fat reduction in food [Lobato-Calleros et al., 2006] and Emulsions and applications │ 8 encapsulation purposes [Mellema et al., 2006; Su et al., 2008]. The preparation of such emulsions is first done with high energy methods to obtain as small as possible inner-emulsions, and afterwards methods that are gentler for the final emulsion are used. The use of gentle methods is done to prevent the ‘inner’ emulsion from coalescing. Other fragilities in this system are risk of oil droplet coalescence, rupture of oil droplets and leakage to and from the inner emulsion [Van Der Graaf et al., 2005]. Membrane emulsion seems to be the best suited emulsion technique for the preparation of the final emulsion. 2.3.2 Nano-emulsions as carriers: nanocapsules Nano-emulsions can be engineered in such a way that they can function as carriers. Aside from being used for improved product features, nano-emulsions can now be used during preparation processes or as nutritional additives. There are many different forms of nano-carriers, including the normal emulsions, double emulsions and encapsulated emulsions. Normal emulsions (O/W and W/O) are the simplest example of a carrier. The desired content can be easily included within the emulsions, provided that the content is miscible with the to-be-dispersed phase. However, most emulsions will not last long in the intestine due to the harsh conditions present there. Therefore, most emulsions are encapsulated with proteins or polymers [Chen et al., 2006a]. There are several different techniques to encapsulate emulsions. One of them is the layer-by-layer technique (LbL). LbL is based on application of oppositely charged molecules [Decher, 1997]. A primary emulsion is made that exhibits a certain charge at the surface of the droplet. Oppositely charged molecules are then added which will adsorb to the droplet surface due to electrostatic attraction. A second layer can be applied with an opposite charge. The cycle can be repeated if necessary. Other encapsulation techniques include the incorporation of the encapsulation-monomers or -proteins in the droplet before emulsion formation. After an initiation signal, usually temperature, the monomers precipitate and migrate to the water/oil interface where polymerisation occurs as a result of a reaction with the water or a component in the water [Tiarks et al., 2001]. An advantage of these encapsulation techniques is that they can be applied after the emulsion has formed. Older methods used solid particles on which the different layers were applied. To use the capsule the core had to be dissolved which required harsh conditions. Removal of the dissolved core is also not always fully achieved. The resulting shell should also be able to open up if it is to be used as a delivery system [Sukhorukov et al., 2004]. Encapsulation of emulsions avoids these problems by loading before the shell is formed. 2.3.3 Nanoliposomes Nanocapsules can also be formed using lipid bilayers, i.e. nanoliposomes. In general, nanoliposomes can be obtained through high- and low-energy techniques. For low energy techniques, liposome generation is based on the PIT method where the addition of water has a temperature that is below the melting point of the surfactant. The addition of water allows for the crystallisation of the shell, thus creating a capsule around the emulsion [Heurtault et al., 2002]. Advantages of nanoliposomes are that they can be readily made from natural or food-grade lipids. They can also be used to target specific areas in the food matrix [Mozafari et al., 2006]. Nanoliposomes are already used as a food processing aid [Piard et al., 1986] and newer applications will mainly be as carriers in the food additives business. More advanced forms of lipid nanocapsules are multi-vesicular lipid capsules. These are similar to the double emulsions but now in the form of liposomes [Mozafari et al., 2006]. Emulsions and applications │ 9 2.3.4 Solid nanoparticles High- and low-energy methods are also produce solid nano-sized particles. This class of structures consists of a homogeneous structure throughout the whole particle whereas the nanocapsules have a (hollow) core-shell structure. Nanoparticles can function as carriers by imbedding the desired substance into the particle itself [Ishihara et al., 2008] or by attaching it to the surface of the particle [Atyabi et al., 2008; Chauvierre et al., 2003]. Production of solid nanoparticles is done by dissolving monomers which are used as the hydrophobic component of the mixture, just like oil is in water. Application of a high-energy method will cause the monomers to polymerise which will then form droplets. Polymerisation is accomplished by molecules that are added to the mixture. These molecules start reacting with the monomers causing them to form polymers [Thickett and Gilbert, 2007]. Droplets are entirely composed of polymerised monomers and are stabilised by surfactants, thereby becoming (solid) nanoparticles. Another type of solid nanoparticles is the solid lipid nanoparticle (SLN). These particles already have a large potential in the pharmaceutical industry as carrier. Production is simple and is usually done with high-energy methods [Vladisavljević and Williams, 2005]. Basically, the lipid is mixed with water or another immiscible fluid at a temperature that is above the melting point of the lipid. The mixture is put through a high-energy device and subsequently cooled down to crystallize the lipids. Advantages of SLN are similar to the solid nanoparticles with respect to carrier capabilities. SLN can be made from lipids which makes them potentially less biotoxic than solid nanoparticles. 2.3.5 Colloidosomes Colloidosomes are emulsions with nanoparticles locked together on the interface forming a shell (fig. 4) [Dinsmore et al., 2002]. Both components (inner-emulsion and shell nanoparticles) can be made from emulsions. Shell formation can be prepared through electrostatic interactions. This preparation can be considered surfactantfree to a certain extent [Sakai, 2008]. Colloidosomes as a whole structure cannot be considered a nanostructure because their size is far greater than 500 nm and can even range into the 10-100 μm. But the nanoparticles that form the shell can be well below 500 nm. Colloidosomes can be, theoretically, multifunctional, with the nanoparticles on the shell as well as the inneremulsion itself acting as a delivery system. However, reports of its use have not yet appeared which is most likely due to this being a recent development. Inner-emulsion Nanoparticle Figure 4 Colloidosome, an advanced product of emulsions. On the left is a schematic view of a colloidosome and on the right scanning electron microscope image of a 10 μm vacuum dried colloidosome. Modified from [Dinsmore et al., 2002]. Emulsions and applications │ 10 2.4 Developments and trends Nanostructures in our food is not a new phenomenon. Food contains many natural nano-sized structures such as globular proteins, one molecule thick layers (e.g. around emulsions) and crystalline lamellae. Such structures are naturally present or formed during production. Size-reduction of emulsions occurred because of attempts to improve food characteristics and not necessarily on the fact that nano-sizing has added advantages. Nanoemulsions may have already been present in our food, especially after the introduction of high pressure homogenisation in the 1950’s [Niro-Soavi, 2008]. The difference between natural nanostructures in our food and the ones discussed in the previous section is that the ones presented here can be engineered to perform certain functions. In other words, the ability to manipulate nano-sized structures for certain tasks is considered to be the innovating part. Nano-emulsions, nanoparticles, lipid nanocapsules and nanofibers are structures that have contributed to the innovation of food ingredients and processing due to specific functions that they could perform. What also can be considered new is the increasing number of methods to generate nanostructures. As nanostructures are being associated with many benefits, several industries are developing new and efficient ways to produce nanostructures. Interestingly, the current methods (high-energy) of producing nano-emulsions are based on old principles and updated techniques. Ultrasound is a relatively new form of production. Ultrasound in the food industry was already used for extraction, viscosity alteration, fermentation, enzyme and microbial inactivation [Patist and Bates, 2008]. Nano-emulsion production is a potentially new candidate to this list. The low-energy methods are also not new. Emulsification through solvent displacement has already been used in the field of radical polymerisation thirty years ago but the mechanism has only recently been described [Vitale and Katz, 2003]. A concern with solvent displacement is that the solvent may not always be fully removed. Nonfood grade ingredients can be used and, if not fully removed, may introduce new risks [Mozafari et al., 2006]. Nanostructures are becoming more complex in order to perform certain functions. Nanostructured coatings, dispersion (e.g. emulsions) and nano-bulk materials such as nanometals, -ceramics and -polymers are fairly simple compared to the structures that are currently being designed. So-called second generation nanostructures are more complex forms of the older nanostructures [Renn and Roco, 2006; Weiss, 2007]. These include the double emulsions, multi vesicular lipid nanocapsules, SLN and colloidosomes. Most of these systems can be distinguished from the first generation by their multi-functional capability and because of this they are most likely capable of more complex interactions during food production and/or in the body [Renn and Roco, 2006; Sanvicens and Marco, 2008]. In summary, nanostructures are naturally present in our food. In the past, attempts to improve food characteristics unintentionally lead to increased nanostructure exposure, whereas nowadays nanostructures are actively introduced in order to improve food characteristics. New methods are being developed to generate increasingly more complex nanostructures. Due to the possibility of more complex interactions of nanostructures extra attention may be needed for their safety assessment, especially in the food industry where EBNS can be used as food additives, processing aids or as a nutritional supplement. Safety assessment of nanostructures in food │ 11 3. Safety assessment of nanostructures in food 3.1 Potential risks Many people have doubts about nanotechnology concerning its safety because so little is known. If nanotechnology is going to be used on a large scale in the food industry then the risks that can be associated with nanostructures will need to be characterised. This section will focus on the identification of possible risks of nanostructures that can be used in the food industry. Possible risks and known effects will be identified, from the preparation methods up to the immune system. In recent years, numerous papers have described the risks of engineered nanomaterials. Examples of these materials are carbon nanotubes, fullerenes and metallic nanoparticles. Also in the pharmaceutical field are there many reviews on risk assessment of nanomaterials and delivery systems. Assessments of nanoformulations used in the food industry, however, have hardly been performed. Due to the lack of food risk assessments of nanostructures, hazard assessments of nanoformulations in the (oral) pharmaceutical research will also be used. 3.1.1 Importance of preparation methods The composition and components of the nanostructures are important determinants of toxicity. A comparison of EBNS made of non-toxic components and potentially toxic components, such as cationic molecules, indicate toxicity related to the materials and not to the fact that it is an EBNS [Weyenberg et al., 2007]. Surfactants are an important component of EBNS, which influence particle size and distribution. High pressure homogenisation and ultrasound generally have a broad size distribution which is often overcome by adding more surfactant. If the surfactant is known to have adverse effects at relevant doses then the preparation method may be a cause for concern. Certain preparation and purification methods can help reduce toxicity mainly by removing excessive amounts [Heydenreich et al., 2003]. One disadvantage is that these preparation methods themselves can affect the size properties of the EBNS, so careful consideration is needed for a balance between risks and benefits. A good preparation can also avoid adverse effects due to impurities. Non-food grade materials are often used to solubilise or dissolve ingredients which may become entrapped together with the active ingredient [Mozafari et al., 2006]. It has been shown in vitro that metal impurities in carbon nanotubes are responsible for the generation of reactive oxidative species and inflammatory reactions in lung cells and macrophages. Purified nanotubes did not display such effects [Pulskamp et al., 2007]. Thus, beside the intrinsic toxicological properties of the components, preparation methods of EBNS are equally important to consider. 3.1.2 Nanostructures in the gastrointestinal tract The gastrointestinal tract (GIT) is the first major contact point with nanostructures. The first large obstacle is the stomach where the high acidity, cleavage enzymes and the mucus layer can prevent nanostructures from entering the body. But this does not provide a full protection. Uptake of various nanostructures was observed throughout the GIT [Desai et al., 1996; Jani et al., 1994; Wang et al., 2006; Wang et al., 2007]. Uptake occurs mostly through specialised membranous epithelial (M) cells in the Peyer’s Patches [Desai et al., 1996; Jani et al., 1994]. Other cells and mechanisms such as enterocytes and paracellular transport, respectively, also make it possible for nanostructures to pass the intestinal epithelium [Lomer et al., 2002]. The GIT is capable of taking up particles of up to 150 μm in size [Hussain et al., 2001], which is well above the size of nanostructures. However, uptake of nanostructures is dependent on the nanostructure and its surface Safety assessment of nanostructures in food │ 12 properties (see subsection 3.1.4). Oral administration of carbon-based nanotubes, fullerenes and quantum dots were found not to be taken up by the intestines and were eventually recovered in the faeces [Deng et al., 2007; Nel et al., 2006], indicating that size is not the main factor driving uptake. Effects of nanostructures on the GIT are not yet fully known. Only slight inflammation of the stomach and intestines in mice was observed after oral administration of nano zinc and titanium dioxide (TiO 2) [Wang et al., 2006; Wang et al., 2007]. In humans, it is thought that exposure to nano-sized structures can contribute to GIT diseases such as inflammatory bowel disease and Crohn’s disease [Lomer et al., 2002]. Other than some inflammation, no other adverse effects of nanostructures in the GIT are known. Detailed hazard characterisations of the GIT and the knowledge of the effects on susceptible people will be essential before engineered nanostructures are allowed to be used in food. 3.1.3 Biodistribution After being taken up in the GIT, nanostructures will distribute throughout the body. Studies of nano TiO 2 and zinc indicate that the liver is the primary site of accumulation [Jani et al., 1994; Sugibayashi et al., 2008; Wang et al., 2006]. Prominent effects are hydropic degeneration and slight necrosis of hepatocytes. Furthermore, increased levels of ALT, AST, ALP, LDH and cholesterol esters indicated liver injury and dysfunction in mice [Wang et al., 2006; Wang et al., 2007]. TiO2, however, is easily eliminated from the body [Sugibayashi et al., 2008]. In a safety evaluation of TiO2 nanoparticles in food most of the TiO2 accumulated in the liver and was gradually eliminated from the body (mice, 30% decrease 1 month after administration). No symptoms or noticeable behaviour changes were observed. Kidneys are the second largest site of accumulation for TiO2 and zinc nanoparticle. Histopathological effects are swelling of the renal glomerulus and proteinic liquid in the renal tubules [Wang et al., 2006; Wang et al., 2007]. Other minor accumulation sites include the spleen, heart and lung tissues. The heart tissue showed no morphological alterations after oral exposure of TiO2 and zinc but blood serum levels of LDH and α-HBDH were higher compared to controls [Wang et al., 2006; Wang et al., 2007]. LDH and α-HBDH are often used as markers of cardiovascular damage. TiO2 and zinc also accumulated in the spleen and lung but no histopathological effects were observed [Jani et al., 1994; Wang et al., 2007]. In summary, the main sites of accumulation are the liver and kidneys where the effects are also the most prominent. Other organs may also be affected to a lesser degree. TiO 2 was shown to be gradually eliminated from the body and thus effects may be reversible. These studies were done with nano TiO2 and zinc but no chronic oral exposure studies of other food nanostructures and EBNS are known. 3.1.4 Effects of surface properties Surface properties of nanostructures are a special issue. First of all, the smaller the particle, the higher its surface-to-volume ratio becomes i.e. smaller nanostructures have relatively more surface area than larger structures. This makes them potentially more reactive. Secondly, the surface can be engineered to exhibit certain properties or functions. The combination of these two aspects is a reason why nanostructures in food raise so many concerns. A few examples of adverse effects due to surface properties are discussed below. An important factor for uptake or internalisation of the particles is surface charge. To enhance the uptake potential of nanostructures their surfaces are often modified with molecules with a certain charge. Particles Safety assessment of nanostructures in food │ 13 [Florence, 1997; Mao et al., 2005] and emulsions [Yang and Benita, 2000] with a positive charge are more easily absorbed by the GIT. The positive charge, however, can have a toxic effect on cells. Positive charged or cationic molecules can enter cells through various cell mechanisms [Xia et al., 2008] or by disrupting the lipid membrane [Leroueil et al., 2008]. A wide variety of commonly used nanostructures was investigated for the potential to create (nano)holes in the lipid bilayer of cells. It seems that regardless of the shape, size, composition, charge density and deformability, all tested cationic nanostructures were capable of disrupting the bilayer by either membrane thinning/erosion, hole formation or expansion [Leroueil et al., 2008]. Hole formation/expansion can lead to leakage of the cell content and even cell death. Whether cell death is attributed to the leakage or to the nanostructure itself is still unclear. Uptake of cationic nanostructures by cells disrupts certain cell processes and leads to cell death. Recently, 60 nm cationic polystyrene nanospheres have been shown in vitro to induce mitochondrial damage in macrophages and bronchial epithelial cells, followed by apoptosis and necrosis, respectively [Xia et al., 2008]. Uptake and cell death mechanisms differed between the two cell lines. Xia et al. also tested polystyrene nanospheres on cells of the liver, adrenal gland and microvascular endothelium. Nanospheres were taken up by these cells but in contrary to macrophages and bronchial epithelial cells, did not induce mitochondrial damage, increased Ca 2+ flux or lysosomal disruption, indicating cell-specific toxicities and mechanisms. The severity of adverse effects can have a charge (as in dose)-response relation. Direct cell-polymer contact assays demonstrated increasing cytotoxicity with increasing charge density of polyesters in mouse fibroblasts [Unger et al., 2007]. Thus, a certain dose-response relationship of nanostructures may be set up based on the charge. The use of cationic molecules as building blocks for nanostructures can still be toxic despite it going through several processes. Processing cationic lipids into 100-500 nm SLN does not reduce their capability to decrease cell viability [Heydenreich et al., 2003]. Though, caution is needed when inferring in vitro results. Comparative studies between in vitro and in vivo pulmonary toxicity results of nanoparticles demonstrated little correlation [Sayes et al., 2007]. Whether this holds true for other non-pulmonary toxicity studies is not known. With such potential toxic effects in mind certain aspects of nanostructures will need extra caution. One technique that deals with surface charge is the LbL technique, which makes it possible to coat emulsions and EBNS [Decher, 1997; Gil et al., 2008]. The application of this technique may unintentionally increase the toxicity of nanostructures and possibly nullifying the intended effect of carrier systems, e.g. reduced toxicity. It is interesting to note that the application of positively charged molecules on nanostructures may decrease oral bioavailability. Mucus in the stomach is negatively charged, and so, positively charged molecules may become entrapped in it [Hoet et al., 2004]. Alternatively, some scientists believe that this can also increase intestinal lifetime and facilitate uptake by presenting nanostructures to the GIT epithelial cells [Arbós et al., 2002; Chen et al., 2006a; Hussain et al., 2001]. This issue is still under debate. Important characteristics of nanostructures, especially surface properties, are to a certain extent accompanied with negative effects. The intestinal environment with its high acidity and cleavage enzymes may change the nanostructure surfaces in a certain way. Inflammatory effects and cell death were induced by pathogen-associated molecular patterns (PAMP) conjugated to food additive-grade nano-TiO2. PAMP may inadvertently adsorb to the surface of TiO 2 [Ashwood et al., 2007]. The gut flora is a potentially large source of PAMPs. Thus, even though a nanostructure can be harmless at first, in or ex vivo events may change its current status. Table 1 summarises a few of the potential risks that can be induced as a result of in or ex vivo (metabolic) processes and interactions. Ultimately, Safety assessment of nanostructures in food │ 14 the proteins that are present or adsorbed to the surface determine what is actually presented to cells [Cedervall et al., 2007]. Nanostructures in the GIT do not necessarily have to be taken up to exert an effect on other organs. One example is the effect on the pH buffering system [Chen et al., 2006b]. Chen et al. investigated the effects of nano- and micro-copper. Severe effects (LD50 was 413 mg/kg body weight for nano-copper as compared to >5000 mg/kg for micro-sized copper) and damage to liver, kidney and spleen where observed. Chen et al. hypothesized that depletion of H+ in the gastric juice due to higher reactivity of nano-copper lead to the formation of bicarbonate ions (HCO3-). HCO3- is hardly excreted by the kidney due to renal disorders and so accumulation occurs that could lead to other metabolic and physiologic states/disorders, such as alkalosis in this case. Table 1 Examples of materials used in food and in nanostructures that can induce undesired effects due to interactions. References are in vivo studies unless indicated otherwise. Material PEG Food use Food additive Possible effect Immune evasion, may enhance unwanted biodistribution Reference [Oberdörster et al., 2005b; Sanvicens and Marco, 2008] Thiamine Natural vitamin in food (e.g. whole cereal grains) Immunogenic, adjuvant properties [Salman et al., 2007] Chitosan Edible films, additive Immune suppression [Roy et al., 1999] Cell membrane damage [Leroueil et al., 2008] (in vitro) Binds with lipopolysaccharide and induces proinflammatory effects and cell death. [Ashwood et al., 2007] (in vitro) Cationic molecules TiO2 Food additive Abbreviations: PEG, polyethylene glycol; TiO2, titanium dioxide. 3.1.5 Effects on the immune system Nanostructures may also affect the immune system in several ways. This can be increased or decreased reactivity, alteration of immune response (e.g. Th1 versus Th2) or adjuvant activity. Nanostructures can be potent adjuvants. With a nanostructure as adjuvant, antigens were able to stimulate the immune system better than when the antigen is presented alone [Salman et al., 2007]. When compared with other adjuvants, including aluminium hydroxide, nanoparticles are capable of inducing higher antibody titres [Khatri et al., 2008; Seferian and Martinez, 2000; Stieneker et al., 1991]. Moreover, smaller nanostructures are associated with higher antibody levels than larger nanostructures [Xiang et al., 2006]. Surface modification of nanostructures can also play a major role. For example, Salman et al. [2007] tested orally administered thiamine-coated and non-coated nanoparticles in mice and showed that the thiamine-coating elicited stronger IgG1 and IgG2a titres. Non-coated nanoparticles induced a typical Th2 response, indicating that coated nanoparticles are capable of shifting towards a more balanced response. This also further indicates the importance of surface properties, i.e. surface can determine functionality of the nanostructure. Nanostructures may also affect diseases based on deregulation of the immune system. Nano metals and ceramics can bias the defence/inflammatory capabilities of macrophages [Lucarelli et al., 2004]. SiO2 nanoparticles biased naive macrophages towards pro-inflammatory reactions by selectively inducing production of IL-1β and TNF-α. Safety assessment of nanostructures in food │ 15 TiO2 was shown to increase expression of viral TLR receptors TLR3 and TLR7. Nanoparticles made from cobalt induced a cytokine profile that resembles experimental and clinical autoimmunity in vitro [Petrarca et al., 2006]. No reports are known that focus on nanostructures in food and the effect on susceptible subjects, e.g., GIT disease models. On the other hand, there are reports describing immune suppression by nanostructures. In a mouse model of peanut allergy, nanoparticles of plasmid DNA and chitosan reduced IgE levels, plasma histamine and vascular leakage. Secretory IgA and serum IgG2a were found after treatment [Roy et al., 1999]. This may imply a positive effect of (functionalised) nanostructures that can be used in food. However, this feature may also raise concerns about undesired and excessive immune suppression. To date there are no extensive reports on this matter, further research is needed. 3.1.3 Other routes of exposure Other routes such as inhalation may become increasingly more relevant as more food applications are going to be developed for nanostructures. Before food ends up on our plates it will undergo several processes. Exposure through inhalation can occur with cooking processes or when the nanostructure is in a powder form. Effects of nanostructures on the respiratory tract have been extensively described [Gill et al., 2007; Mühlfeld et al., 2008; Oberdörster et al., 2005b]. Interestingly, it was already known sixty years ago that nanostructures (<500 nm) can be taken up and transported up the olfactory bulb and nerve [Oberdörster et al., 2005b]. Oberdörster et al. [2005] noted that these studies were performed on rodents and questioned the relevance to humans. Indeed, the human olfactory mucosa comprises only 5% of the total nasal mucosal surface as opposed to 50% in rats. Thus, relative exposure would seem to be far less for humans. Nevertheless, this still may be a concern for people with pets. Other important effects on the lungs are, in general, inflammation and fibrosis, generation of reactive oxidative species, various effects on macrophages and cardiovascular effects. Again, surface properties can have a large influence [Oberdörster et al., 2005b]. Most of the assessment studies have used metallic or carbon-based nanostructures and not much emphasis is given to EBNS that are used in the pharmaceutical or food industry. More research is needed using EBNS that can also be used in the food industry. A third major route of potential uptake is through dermal exposure. Skin contact with nanostructures (in food) can occur during handling or preparation of food. Risk assessments of cosmetics, mainly sunscreens and lotions, have shown however, that nanostructures do not penetrate the living skin [Nohynek et al., 2007]. Moreover, it was concluded that nano-sized structures do not significantly penetrate deeper than micro-sized structures, although the definition of micro-sized structures was not clearly explained by Nohynek et al. 3.2 A food-specific risk assessment In 2007 the EU Scientific Committee on Emerging and Newly-Identified Health Risks (SCENIHR) developed a tiered risk assessment methodology for nanomaterials based on an exposure assessment algorithm (fig. 5) [SCENIHR, 2007]. The SCENIHR acknowledges that both assessments deal with nanostructures in general and that there may be a need for more industry-specific risk assessments. Here, several aspects will be discussed that can make the exposure assessment algorithm by the SCENIHR more specific to the food industry. Safety assessment of nanostructures in food │ 16 Figure 5 Exposure assessment algorithm from SCENIHR [SCENIHR, 2007]. Several of the guiding questions proposed by the SCENIHR in figure 5 are directed to nanostructures not intended for food. For example, in the SCENIHR report nanostructures do not need to be considered as such if they are found to be soluble in water. However, water solubility is an important aspect for uptake in the GIT [Bronner, 1993]. Many nutrients are required to be soluble in water or oil in order to be taken up efficiently. Nutrients that are insoluble in water and/or oil such as β-carotene can be delivered with nanostructures that have been made soluble [Yuan et al., 2008]. Thus, water soluble as well as water insoluble nanostructures need to be considered in food risk assessments. Food nanostructures larger than 100 nm should also be considered in risk assessments. Nanoparticles in the air can penetrate deeper into the lungs depending on their size. Nanostructures in food do not have this limitation, making it easier to come in contact with cells. Though, a proper upper limit still needs to be determined. Another physicochemical property, agglomeration, may be a potential concern. Agglomeration (or coalescence) can cause nano-emulsions to lose their beneficial effects. Food manufacturers can avoid this by applying molecules capable of repulsing other particles [Studart et al., 2007]. However, such procedures can alter other properties of the nanostructures [Kirchner et al., 2005] and in vivo behaviours (e.g. increased circulation capabilities) [Garnett and Kallinteri, 2006], consequently introducing new risks. In food risk assessments it may therefore be important to assess the effect of anti-agglomeration measures. One important aspect of nanotechnology in the food industry is that there can be an intended exposure. Currently, manufacturers try to minimise the amount of processing and enhance food nutrition. For instance, by reducing the transport medium the uptake of nutrients in the gastrointestinal tract will be improved [Desai et al., 1997; Jani et al., 1990]. Safety assessment of nanostructures in food │ 17 There are two types of nanostructures with an intended exposure: 1) nanostructures which are involved in nutritional enhancement. This is mostly in the form of carrier systems. The goal of such nanostructures is to have a high as possible bioavailability. These structures will concern both occupational and consumer safety. 2) Nanostructures which are present in the end-product but have a food-improvement or cosmetic related function such as enhancement of taste or smell. This also includes food spoilage and antimicrobial protection [An et al., 2008]. These nanostructures should not be taken up into the body. This may raise concerns for manufacturers. Nano fragrance molecules, for example, can be taken up by cells in the lung and nasal cavity [Oberdörster et al., 2005b]. Bioavailability of these nanostructures should be as low as possible. Nanostructures that do not have any intended exposures will most likely be used as processing aids or as a functionalised coating on equipment. This can be for example nanocapsules protecting an enzyme during the production process and/or allowing gradual release of the enzyme afterwards [Piard et al., 1986]. When these nanostructures remain in the end-product, measures may be necessary to insure that consumer exposure (and bioavailability) is kept at a minimum, especially when adverse effects are known. In vitro testing is well suited as an initial screening for adverse effects but a recent comparison of in vitro and in vivo pulmonary tests indicated little correlation [Sayes et al., 2007]. Characteristics of nanostructures (e.g. size, size distribution, charge etc.) can be altered by the food matrix, processing of the food matrix and the GIT. To describe the effects as accurately as possible, in vivo tests need to be carried out with the matrices that (will) house the nanostructures [Oberdörster et al., 2005a]. Although, with this approach many complications arise that may obscure the effects of the nanostructure. Feeding high levels of whole foods to test animals can lead to nutritional imbalances and difficulties with establishing causal relations [Constable et al., 2007]. To reduce unnecessary animal testing in vitro studies should get priority over in vivo studies. If in vitro studies indicate adverse effects at relevant doses, then in vivo experiments should be done to confirm results. In vitro and in vivo studies need to be developed that correlate with each other. Effects of chronic exposure are harder to determine than acute effects. To provide a certain level of safety on the long term, measures have been devised that take into account a lifetime exposure. One measure discussed by the SCENIHR is the threshold of toxicological concern (TTC). It provides guidance in the form of a level of exposure or intake per person per day below which no significant risk to humans is expected to exist. The determination of a TTC value is done by comparing the molecular structure with other structural analogues and the knowledge gained from the general toxicity database from the past sixty years [Kroes et al., 2005; Kroes and Kozianowski, 2002; Munro et al., 2008]. This method is beneficial with respect to limited resources, time, and expertise and is very useful when there is little or no data available of the substance. For nanostructures, however, the TTC may not be an appropriate guidance value. Nanomaterials are a relatively new phenomenon, and so, there are no other substances to compare with. If nanostructures are made from existing components such as chitosan then a TTC value can be derived from chitosan toxicity (bulk) data. But the effect of nano-sizing chitosan is not known and therefore some minimal amount of (in vivo) testing may still be required. Also, due to their complexity different forms of nanostructures can present different risks depending on their manufacturing process [Lin, 2007]. The lack of knowledge of nanostructures will make it difficult to apply a TTC concept in risk assessments. Knowledge on nanostructure characteristics and preferably also dose-response relations will therefore need to be generated first. Because comparison with structural analogues may not always be possible, an alternative to the TTC may be needed. The acceptable daily intake (ADI) may fulfil this role for now. The ADI is also a guidance value for the daily intake of a substance over the entire lifetime [Lu, 1988]. The difference between TTC and ADI is that the Safety assessment of nanostructures in food │ 18 ADI relies on identification of the highest dose level where no effect occurs and subsequent application of a safety margin, whereas the TTC assures safety based on sufficiently low exposure (in the absence of specific toxicity data) [Munro et al., 2008]. Thus, the advantage of an ADI over the TTC is that it is more specific to a substance. The ADI is likely to be a precursor to the TTC because it is derived after the determination of adverse effects. The TTC is more of a predictive form of hazard assessment and can be more efficient than the ADI if the nanostructure is composed of structures with already existing toxicity data. ADI values are currently being used for food additives and judging of the potentials of nanostructures, the first applications will most likely be as food additives. One issue that may need addressing in the future but is not explicitly mentioned in the SCENIHR report is the possibility of multi-functionality. The currently known nanostructures (nanotubes, metallic nanoparticles) are relatively simple but future nanostructures are expected to be more complex (delivery systems; e.g. colloidosomes, fig. 6) [Renn and Roco, 2006; Sanvicens and Marco, 2008; Weiss, 2007]. Renn and Roco [2006] predict that the next generation of nanostructures will involve assembly and networking systems, adding up to complexity and multi-functionality. In nano-food processing this can possibly include miniature ‘factories’ capable of several (enzymatic) processing steps at the same time. But should we therefore be afraid of ‘multitoxicity’? This will most likely depend on the nanostructure capabilities. It may be that the safety of multifunctional nanostructures will have to be judged on each separate function and its capability as a whole. Figure 6 Example of a fictitious multifunctional nanostructure. Figure from [Sanvicens and Marco, 2008] Laws have been implemented to ensure food safety. But current food laws may have unintentionally created opportunities for manufactures to circumvent testing of nanostructures. This mainly concerns nanostructures that have been made through a top-down approach. Existing substances have usually already been through extensive testing, but in all cases the effect of size has not been considered. Therefore, nanostructures can be unjustly deemed safe due to the existence of a Safety Data Sheet of its bulk form. TiO 2 is used as a whiting agent in food and was recently deemed safe for use according to EU legislation [HSE, 2006]. Such regulations may unintentionally help nanostructures and manufacturers circumvent nano-specific risk assessments. This can be a concern if adverse effects are size-dependent. Figure 7 proposes a food nanotechnology risk assessment paradigm that incorporates the aspects discussed above. A sequence of questions is asked to determine the need for further extensive testing. This paradigm builds on the existing risk assessment by the SCENIHR (fig. 5) and incorporates several of its elements. Furthermore, this Safety assessment of nanostructures in food │ 19 paradigm is only intended for nanostructures that are in contact with food and does not address environmental issues. The risk assessments set up by the SCENIHR focuses more on physicochemical properties as a rationale for safety testing (fig. 5). The proposed risk assessment, on the other hand, starts by identifying the purpose of the nanostructure (i.e. intended versus unintended). If a nanostructure is only used during food production and removed afterwards from the end-product (i.e. unintended), then no detailed assessments are needed (Fig 7, left column). This is to prevent unnecessary and costly testing. The only other concern of these types of nanostructures is limited to occupational safety. For nanostructures that remain in the end-product, further testing may depend on assessment of the known data on the physicochemical properties (fig.7, second column). This is a similar procedure as in figure 5. Enhanced solubility or reduced agglomeration potential can increase bioavailability. These and other properties can give a good indication of the bioavailability potential without doing any extensive testing. Alterations to the nanostructure throughout its life should also be taken into consideration. This includes physical changes but also adsorption of uptake enhancing molecules. A changing environment like the GIT or (domestic) heating can change such properties and can even create different routes of exposure. Therefore also other routes such as inhalation should be considered. Besides adverse effects due to uptake, effects not related to uptake such as irritation or inflammation may also need to be considered. Nanostructures with high bioavailability should be subject to at least some (initial) tests to ensure a minimum level of consumer safety (fig. 7, third column). The initial testing should also include further characterisation of other unknown physicochemical properties. Effects can be compared to the corresponding bulk material. Bulk material guidelines can be followed only when effects are of the same kind and less severe at the same mass concentration. This is to avoid manufacturers bypassing further testing as a result of existing bulk form data. Gaps in the legislation should not be a reason to avoid testing even though the bulk form of the nanostructure has been in use for a long time (e.g. GRAS, history of safe use). A full risk assessment should be performed when adverse effects of the nanostructure are more severe than the bulk form or when tests indicate different (new) kinds of adverse effects. Safety assessment of nanostructures in food │ 20 Is there an intended exposure? YES NO Will the nanostructure be removed from the end-product? NO YES NO/ Unknown YES Is or can the nanostructure be fully contained during production? Intended exposure but no/low bioavailability? YES Does the nanostructure or its components have a GRAS-status or a history of safe use (not counting in as bulk form)?* NO NO/ Unknown YES Based on physicochemical properties and/or structural features, is there potential for uptake? NO YES/ Unknown No further tests may be necessary. NO Initial tests: in vitro studies. Are there any adverse effects? YES Are the nanostructures also in bulk form? Is there uptake possible due to alterations by the food matrix, processing of the food matrix and/or GIT? NO NO YES YES YES/ Unknown NO No further tests may be necessary. Intended exposure with high bioavailability YES YES Validate initial tests with specialized in vitro, in vivo and ADME studies. Are effects similar/worse than in initial tests? Are there any other exposure routes possible? NO YES No further tests may be necessary. Do in vivo effects occur at relevant doses? Are effects more severe than corresponding bulk material? NO Guidelines of the bulk material may suffice. NO NO No further tests may be necessary. YES Derive ADI or, if possible, TTC YES Are there any other exposure routes possible? NO Complete risk assessment Figure 7 Proposed risk assessment paradigm for nanostructures that can be used in the food industry. A series of questions are asked of which the answers determine the need for further studies. *The large box about GRAS-status or a history of safe use should not be considered as an interconnecting box between the second and third column but instead as two separate boxes with similar content. This box was depicted as one large box solely for graphical reasons. Abbreviations: GRAS, generally recognised as safe; GIT, gastrointestinal tract; ADME, absorption, distribution, metabolism and excretion; ADI, acceptable daily intake; TTC threshold of toxicological concern. Conclusions │ 21 4. Conclusions One of the purposes of this report was to investigate the possibilities to generate various nanostructures through emulsification methods that can be used in the food industry. Methods to produce nanostructures are based on old techniques and principles. Manufacturers are developing more efficient methods to produce EBNS, thereby increasing consumer exposure. A wide variety of EBNS can be obtained through emulsification. These nanostructures can be functionalised to perform certain functions such as enzyme delivery. This aspect differentiates engineered nanostructures from natural nanostructures which are already present in food in the form of proteins, bacteria, single molecules (e.g. vitamins) etc. Nanostructures that are currently being developed are becoming more complex which enables them to perform multiple functions. These multi-functional EBNS may require special attention in risk assessments. The other purpose and main focus of this report was to identify possible risks that may arise due to nanostructures in food and to determine important aspects for a risk assessment that is specific to food nanostructures. Surface properties are probably the most important aspect of nanostructures to consider. Surface molecules can determine the fate, function and possible risks of nanostructures. There is still very little known on the risks of nanostructures, especially when it comes to effects on the GIT. There have been a lot of in vitro studies conducted and relatively few (oral) in vivo studies. Pulmonary toxicity studies of nanoparticles indicated little correlation. Similar investigations need to be conducted for other non-pulmonary studies. Also, most studies have used metallic or carbon-based nanoparticles and no extensive studies have been conducted using other kinds of nanostructures. Effect of nanostructures on hypersensitivity and disease models (as susceptible subjects) will also need more research. Recommendations for a food nanostructures-specific risk assessment are: 1) to base assessments on the purpose of the nanostructure i.e. intended versus unintended exposure. Some nanostructures are intended to be taken up whereas others are only used during production, which does not need extensive testing. 2) The need for testing based on physicochemical properties differs for nanostructures in food compared to those used in other industries. Solubility can increase uptake and, depending on the purpose may be undesired. Assessments should also include nanostructures larger than 100 nm because cells are capable of taking up larger sized nanostructures. Nanostructures can be ‘engineered’ with certain properties which may have similar adverse effects as smaller sized nanostructures. 3) A minimum amount of testing for nanostructures with a potential of being taken up (based on physicochemical properties), whether it is intended or not. This is to provide a minimum level of safety. Further testing if needed, can provide guidance values such as ADI and may even set up a toxicity database that can be used for other nanostructures. Such a database will be helpful for a TTC concept, which may help in further reducing (animal) testing. Implementation of these recommendations in a risk assessment will make it more specific to for nanostructures in food. The risk assessment paradigm presented in figure 7 incorporates these recommendations in the form of a series of questions of which the answers determine the need for further testing. References │ 22 5. References An, J., M. Zhang, S. Wang, and J. Tang (2008). Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT - Food Science and Technology 41:1100-1107. Anton, N., J. P. Benoit, and P. Saulnier (2008). Design and production of nanoparticles formulated from nano-emulsion templates-A review. Journal of Controlled Release 128:185-199. Arbós, P., M. A. Arangoa, M. A. Campanero, and J. M. Irache (2002). Quantification of the bioadhesive properties of protein-coated PVM/MA nanoparticles. International Journal of Pharmaceutics 242:129136. Ashwood, P., R. P. H. Thompson, and J. J. Powell (2007). Fine particles that adsorb lipopolysaccharide via bridging calcium cations may mimic bacterial pathogenicity towards cells. Experimental Biology and Medicine 232:107-117. Atyabi, F., F. A. Moghaddam, R. Dinarvand, M. J. Zohuriaan-Mehr, and G. Ponchel (2008). Thiolated chitosan coated poly hydroxyethyl methacrylate nanoparticles: Synthesis and characterization. Carbohydrate Polymers 74:59-67. Branen, J. R., M. J. Hass, E. R. Douthit, W. C. Maki, and A. L. Branen (2007). Detection of Escherichia coli O157, Salmonella enterica serovar Typhimurium, and staphylococcal enterotoxin B in a single sample using enzymatic bio-nanotransduction. Journal of Food Protection 70:841-850. Bronner, F. (1993). Nutrient bioavailability, with special reference to calcium. Journal of Nutrition 123:797-802. Cedervall, T., I. Lynch, S. Lindman, T. Berggård, E. Thulin, H. Nilsson, K. A. Dawson, and S. Linse (2007). Understanding the nanoparticle-protein corona using methods to quntify exchange rates and affinities of proteins for nanoparticles. Proceedings of the National Academy of Sciences of the United States of America 104:2050-2055. Charcosset, C., I. Limayem, and H. Fessi (2004). The membrane emulsification process - A review. Journal of Chemical Technology and Biotechnology 79:209-218. Chauvierre, C., D. Labarre, P. Couvreur, and C. Vauthier (2003). Novel Polysaccharide-Decorated Poly(Isobutyl Cyanoacrylate) Nanoparticles. Pharmaceutical Research 20:1786-1793. Chen, L., G. E. Remondetto, and M. Subirade (2006a). Food protein-based materials as nutraceutical delivery systems. Trends in Food Science and Technology 17:272-283. Chen, Z., H. Meng, G. Xing, C. Chen, Y. Zhao, G. Jia, T. Wang, H. Yuan, C. Ye, F. Zhao, Z. Chai, C. Zhu, X. Fang, B. Ma, and L. Wan (2006b). Acute toxicological effects of copper nanoparticles in vivo. Toxicology Letters 163:109-120. Constable, A., D. Jonas, A. Cockburn, A. Davi, G. Edwards, P. Hepburn, C. Herouet-Guicheney, M. Knowles, B. Moseley, R. Oberdörfer, and F. Samuels (2007). History of safe use as applied to the safety assessment of novel foods and foods derived from genetically modified organisms. Food and Chemical Toxicology 45:2513-2525. Daufin, G., J. P. Escudier, H. Carrère, S. Bérot, L. Fillaudeau, and M. Decloux (2001). Recent and emerging applications of membrane processes in the food and dairy industry. Food and Bioproducts Processing: Transactions of the Institution of of Chemical Engineers, Part C 79:89-102. Decher, G. (1997). Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 277:1232-1237. Deng, X., G. Jia, H. Wang, H. Sun, X. Wang, S. Yang, T. Wang, and Y. Liu (2007). Translocation and fate of multi-walled carbon nanotubes in vivo. Carbon 45:1419-1424. Desai, M. P., V. Labhasetwar, G. L. Amidon, and R. J. Levy (1996). Gastrointestinal uptake of biodegradable microparticles: Effect of particle size. Pharmaceutical Research 13:1838-1845. Desai, M. P., V. Labhasetwar, E. Walter, R. J. Levy, and G. L. Amidon (1997). The mechanism of uptake of biodegradable microparticles in Caco-2 cells is size dependent. Pharmaceutical Research 14:15681573. References │ 23 Dinsmore, A. D., M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch, and D. A. Weitz (2002). Colloidosomes: Selectively permeable capsules composed of colloidal particles. Science 298:1006-1009. Erlat, A. G., B. M. Henry, J. J. Ingram, D. B. Mountain, A. McGuigan, R. P. Howson, C. R. M. Grovenor, G. A. D. Briggs, and Y. Tsukahara (2001). Characterisation of aluminium oxynitride gas barrier films. Thin Solid Films 388:78-86. Florence, A. T. (1997). The oral absorption of micro- and nanoparticulates: Neither exceptional nor unusual. Pharmaceutical Research 14:259-266. Garnett, M. C., and P. Kallinteri (2006). Nanomedicines and nanotoxicology: Some physiological principles. Occupational Medicine 56:307-311. Gil, P. R., L. L. del Mercato, P. del Pino, A. Muñoz-Javier, and W. J. Parak (2008). Nanoparticle-modified polyelectrolyte capsules. Nano Today 3:12-21. Gill, S., R. Löbenberg, T. Ku, S. Azarmi, W. Roa, and E. J. Prenner (2007). Nanoparticles: Characteristics, mechanisms of action, and toxicity in pulmonary drug delivery - A review. Journal of Biomedical Nanotechnology 3:107-119. Graveland-Bikker, J. F., and C. G. de Kruif (2006). Unique milk protein based nanotubes: Food and nanotechnology meet. Trends in Food Science and Technology 17:196-203. Heurtault, B., P. Saulnier, B. Pech, J. E. Proust, and J. P. Benoit (2002). A novel phase inversion-based process for the preparation of lipid nanocarriers. Pharmaceutical Research 19:875-880. Heydenreich, A. V., R. Westmeier, N. Pedersen, H. S. Poulsen, and H. G. Kristensen (2003). Preparation and purification of cationic solid lipid nanospheres - Effects on particle size, physical stability and cell toxicity. International Journal of Pharmaceutics 254:83-87. Hoet, P. H. M., I. Brüske-Hohlfeld, and O. V. Salata (2004). Nanoparticles - Known and unknown health risks. Journal of Nanobiotechnology 2. HSE. (2006). Review of the adequacy of current regulatory regimes to secure effective regulation of nanoparticles created by nanotechnology. Health and Safety Executive. (Available online at http://www.hse.gov.uk/horizons/nanotech/regulatoryreview.pdf) Hussain, N., V. Jaitley, and A. T. Florence (2001). Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Advanced Drug Delivery Reviews 50:107-142. Ishihara, T., M. Takahashi, M. Higaki, M. Takenaga, T. Mizushima, and Y. Mizushima (2008). Prolonging the in vivo residence time of prostaglandin E1 with biodegradable nanoparticles. Pharmaceutical Research 25:1686-1695. Jani, P., G. W. Halbert, J. Langridge, and A. T. Florence (1990). Nanoparticle uptake by the rat gastrointestinal mucosa: Quantitation and particle size dependency. Journal of Pharmacy and Pharmacology 42:821-826. Jani, P. U., D. E. McCarthy, and A. T. Florence (1994). Titanium dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. International Journal of Pharmaceutics 105:157-168. Johnston, J. H., J. E. Grindrod, M. Dodds, and K. Schimitschek (2008). Composite nano-structured calcium silicate phase change materials for thermal buffering in food packaging. Current Applied Physics 8:508-511. Joscelyne, S. M., and G. Trägårdh (1999). Food emulsions using membrane emulsification: Conditions for producing small droplets. Journal of Food Engineering 39:59-64. Kampers, F. W. H. (2007). Food Nanoscience in the Netherlands. http://www.worldfoodscience.org/cms/?pid=1004071 (Accessed 11 August 2008). Available at: Khatri, K., A. K. Goyal, P. N. Gupta, N. Mishra, and S. P. Vyas (2008). Plasmid DNA loaded chitosan nanoparticles for nasal mucosal immunization against hepatitis B. International Journal of Pharmaceutics 354:235-241. References │ 24 Kirchner, C., T. Liedl, S. Kudera, T. Pellegrino, A. M. Javier, H. E. Gaub, S. Stölzle, N. Fertig, and W. J. Parak (2005). Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Letters 5:331-338. Kroes, R., J. Kleiner, and A. Renwick (2005). The threshold of toxicological concern concept in risk assessment. Toxicological Sciences 86:226-230. Kroes, R., and G. Kozianowski (2002). Threshold of toxicological concern (TTC) in food safety assessment. Toxicology Letters 127:43-46. Leroueil, P. R., S. A. Berry, K. Duthie, G. Han, V. M. Rotello, D. Q. McNerny, J. R. Baker Jr, B. G. Orr, and M. M. B. Holl (2008). Wide varieties of cationic nanoparticles induce defects in supported lipid bilayers. Nano Letters 8:420-424. Li, D., and Y. Xia (2004). Electrospinning of nanofibers: Reinventing the wheel? Advanced Materials 16:11511170. Lin, A. C. (2007). Size matters: Regulating nanotechnology. Harvard Environmental Law Review 31:349-408. Lobato-Calleros, C., E. Rodriguez, O. Sandoval-Castilla, E. J. Vernon-Carter, and J. Alvarez-Ramirez (2006). Reduced-fat white fresh cheese-like products obtained from W1/O/W2 multiple emulsions: Viscoelastic and high-resolution image analyses. Food Research International 39:678-685. Lomer, M. C. E., R. P. H. Thompson, and J. J. Powell (2002). Fine and ultrafine particles of the diet: Influence on the mucosal immune response and association with crohn's disease. Proceedings of the Nutrition Society 61:123-130. Lu, F. C. (1988). Acceptable daily intake: inception, evolution and application. Regulatory Toxicology and Pharmacology 8:45-60. Lucarelli, M., A. M. Gatti, G. Savarino, P. Quattroni, L. Martinelli, E. Monari, and D. Boraschi (2004). Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles. European Cytokine Network 15:339-346. Mao, S., O. Germershaus, D. Fischer, T. Linn, R. Schnepf, and T. Kissel (2005). Uptake and transport of PEG-graft-trimethyl-chitosan copolymer-insulin nanocomplexes by epithelial cells. Pharmaceutical Research 22:2058-2068. Meleson, K., S. Graves, and T. G. Mason (2004). Formation of concentrated nanoemulsions by extreme shear. Soft Materials 2:109-123. Mellema, M., W. A. J. Van Benthum, B. Boer, J. Von Harras, and A. Visser (2006). Wax encapsulation of water-soluble compounds for application in foods. Journal of Microencapsulation 23:729-740. Mozafari, M. R., J. Flanagan, L. Matia-Merino, A. Awati, A. Omri, Z. E. Suntres, and H. Singh (2006). Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. Journal of the Science of Food and Agriculture 86:2038-2045. Mühlfeld, C., B. Rothen-Rutishauser, F. Blank, D. Vanhecke, M. Ochs, and P. Gehr (2008). Interactions of nanoparticles with pulmonary structures and cellular responses. American journal of physiology. Lung cellular and molecular physiology 294. Munro, I. C., A. G. Renwick, and B. Danielewska-Nikiel (2008). The Threshold of Toxicological Concern (TTC) in risk assessment. Toxicology Letters 180:151-156. Nel, A., T. Xia, L. Mädler, and N. Li (2006). Toxic potential of materials at the nanolevel. Science 311:622627. Niro-Soavi (2008). History of Niro Soavi. Available at: soavi.com/soavi/cmsdoc.nsf/WebDoc/ndkw73ejht (Accessed 17 June 2008). NNI (2008). National Nanotechnology Initiative funding. http://www.nano.gov/html/about/funding.html (Accessed 10 August 2008). http://www.niro- Available at: Nohynek, G. J., J. Lademann, C. Ribaud, and M. S. Roberts (2007). Grey Goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Critical Reviews in Toxicology 37:251-277. References │ 25 Oberdörster, G., A. Maynard, K. Donaldson, V. Castranova, J. Fitzpatrick, K. Ausman, J. Carter, B. Karn, W. Kreyling, D. Lai, S. Olin, N. Monteiro-Riviere, D. Warheit, and H. Yang (2005a). Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Particle and Fibre Toxicology 2. Oberdörster, G., E. Oberdörster, and J. Oberdörster (2005b). Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives 113:823-839. Patist, A., and D. Bates (2008). Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innovative Food Science and Emerging Technologies 9:147-154. Petrarca, C., A. Perrone, N. Verna, F. Verginelli, J. Ponti, E. Sabbioni, L. Di Giampaolo, V. Dadorante, C. Schiavone, P. Boscolo, R. Mariani Costantini, and M. Di Gioacchino (2006). Cobalt nanoparticles modulate cytokine in vitro release by human mononuclear cells mimicking autoimmune disease. International journal of immunopathology and pharmacology 19:11-14. Piard, J. C., M. El Soda, and W. Alkhalaf (1986). Acceleration of cheese ripening with liposome- entrapped proteinase. Biotechnology Letters 8:241-246. Pinnamaneni, S., N. G. Das, and S. K. Das (2003). Comparison of oil-in-water emulsions manufactured by microfluidization and homogenization. Pharmazie 58:554-558. Powell, M. C., M. P. A. Griffin, and S. Tai (2008). Bottom-Up Risk Regulation? How Nanotechnology Risk Knowledge Gaps Challenge Federal and State Environmental Agencies. Environmental Management:118. Pulskamp, K., S. Diabate, and H. F. Krug (2007). Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicology Letters 168:58-74. Ramakrishna, S., K. Fujihara, W. E. Teo, T. Yong, Z. Ma, and R. Ramaseshan (2006). Electrospun nanofibers: Solving global issues. Materials Today 9:40-50. Renn, O., and M. C. Roco (2006). Nanotechnology and the need for risk governance. Journal of Nanoparticle Research 8:153-191. Rhim, J. W., S. I. Hong, H. M. Park, and P. K. W. Ng (2006). Preparation and characterization of chitosanbased nanocomposite films with antimicrobial activity. Journal of Agricultural and Food Chemistry 54:5814-5822. Rodríguez Patino, J. M., C. Carrera Sánchez, and M. R. Rodríguez Niño (2008). Implications of interfacial characteristics of food foaming agents in foam formulations. Advances in Colloid and Interface Science 140:95-113. Roy, K., H. Q. Mao, S. K. Huang, and K. W. Leong (1999). Oral gene delivery with chitosan-DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nature Medicine 5:387-391. Sakai, T. (2008). Surfactant-free emulsions. Current Opinion in Colloid and Interface Science 13:228-235. Salman, H. H., C. Gamazo, M. Agüeros, and J. M. Irache (2007). Bioadhesive capacity and immunoadjuvant properties of thiamine-coated nanoparticles. Vaccine 25:8123-8132. Sanguansri, P., and M. A. Augustin (2006). Nanoscale materials development - a food industry perspective. Trends in Food Science and Technology 17:547-556. Sanvicens, N., and M. P. Marco (2008). Multifunctional nanoparticles - properties and prospects for their use in human medicine. Trends in Biotechnology 26:425-433. Sayes, C. M., K. L. Reed, and D. B. Warheit (2007). Assessing toxicology of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicological Sciences 97:163180. SCENIHR (2007). Opinion on the appropriateness of the risk assessment methodology in accordance with the technical guidance documents for new and existing substances for assessing the risks of nanomaterials, 22 June 2007. Available online at: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_010.pdf References │ 26 Schultz, S., G. Wagner, K. Urban, and J. Ulrich (2004). High-pressure homogenization as a process for emulsion formation. Chemical Engineering and Technology 27:361-368. Seeman, N. C., and A. M. Belcher (2002). Emulating biology: Building nanostructures from the bottom up. Proceedings of the National Academy of Sciences of the United States of America 99:6451-6455. Seferian, P. G., and M. L. Martinez (2000). Immune stimulating activity of two new chitosan containing adjuvant formulations. Vaccine 19:661-668. Solans, C., P. Izquierdo, J. Nolla, N. Azemar, and M. J. Garcia-Celma (2005). Nano-emulsions. Current Opinion in Colloid and Interface Science 10:102-110. Sorrentino, A., G. Gorrasi, and V. Vittoria (2007). Potential perspectives of bio-nanocomposites for food packaging applications. Trends in Food Science and Technology 18:84-95. Stieneker, F., J. Kreuter, and J. Lower (1991). High antibody titres in mice with polymethylmethacrylate nanoparticles as adjuvant for HIV vaccines. AIDS 5:431-435. Studart, A. R., E. Amstad, and L. J. Gauckler (2007). Colloidal stabilization of nanoparticles in concentrated suspensions. Langmuir 23:1081-1090. Su, J., J. Flanagan, and H. Singh (2008). Improving encapsulation efficiency and stability of water-in-oil-inwater emulsions using a modified gum arabic (Acacia (sen) SUPER GUM™). Food Hydrocolloids 22:112-120. Sugibayashi, K., H. Todo, and E. Kimura (2008). Safety evaluation of titanium dioxide nanoparticles by their absorption and elimination profiles. Journal of Toxicological Sciences 33:293-298. Sukhorukov, G. B., A. Fery, M. Brumen, and H. Möhwald (2004). Physical chemistry of encapsulation and release. Physical Chemistry Chemical Physics 6:4078-4089. Tadros, T., P. Izquierdo, J. Esquena, and C. Solans (2004). Formation and stability of nano-emulsions. Advances in Colloid and Interface Science 108-109:303-318. Thickett, S. C., and R. G. Gilbert (2007). Emulsion polymerization: State of the art in kinetics and mechanisms. Polymer 48:6965-6991. Thomas, T., K. Thomas, N. Sadrieh, N. Savage, P. Adair, and R. Bronaugh (2006). Research strategies for safety evaluation of nanomaterials, part VII: Evaluating consumer exposure to nanoscale materials. Toxicological Sciences 91:14-19. Tiarks, F., K. Landfester, and M. Antonietti (2001). Preparation of polymeric nanocapsules by miniemulsion polymerization. Langmuir 17:908-918. Unger, F., M. Wittmar, and T. Kissel (2007). Branched polyesters based on poly[vinyl-3(dialkylamino)alkylcarbamate-co-vinyl acetate-co-vinyl alcohol]-graft-poly(d,l-lactide-co-glycolide): Effects of polymer structure on cytotoxicity. Biomaterials 28:1610-1619. Van Der Graaf, S., C. G. P. H. Schroën, and R. M. Boom (2005). Preparation of double emulsions by membrane emulsification - A review. Journal of Membrane Science 251:7-15. Vega-Villa, K. R., J. K. Takemoto, J. A. Yáñez, C. M. Remsberg, M. L. Forrest, and N. M. Davies (2008). Clinical toxicities of nanocarrier systems. Advanced Drug Delivery Reviews 60:929-938. Vincze, I., and G. Vatai (2004). Application of nanofiltration for coffee extract concentration. Desalination 162:287-294. Vitale, S. A., and J. L. Katz (2003). Liquid droplet dispersions formed by homogeneous liquid-liquid nucleation: "The ouzo effect". Langmuir 19:4105-4110. Vladisavljević, G. T., and R. A. Williams (2005). Recent developments in manufacturing emulsions and particulate products using membranes. Advances in Colloid and Interface Science 113:1-20. Wang, B., W. Y. Feng, T. C. Wang, G. Jia, M. Wang, J. W. Shi, F. Zhang, Y. L. Zhao, and Z. F. Chai (2006). Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicology Letters 161:115-123. References │ 27 Wang, J., G. Zhou, C. Chen, H. Yu, T. Wang, Y. Ma, G. Jia, Y. Gao, B. Li, J. Sun, Y. Li, F. Jiao, Y. Zhao, and Z. Chai (2007). Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicology Letters 168:176-185. Weiss, J. (2007). State of food nanotechnology in the United States in 2007. http://www.worldfoodscience.org/cms/?pid=1004045 (Accessed 12 August 2008). Available at: Weyenberg, W., P. Filev, D. Van den Plas, J. Vandervoort, K. De Smet, P. Sollie, and A. Ludwig (2007). Cytotoxicity of submicron emulsions and solid lipid nanoparticles for dermal application. International Journal of Pharmaceutics 337:291-298. Windhab, E. J., M. Dressler, K. Feigl, P. Fischer, and D. Megias-Alguacil (2005). Emulsion processing From single-drop deformation to design of complex processes and products. Chemical Engineering Science 60:2101-2113. WWICS (2008). Internet survey by Woodrow Wilsion Institute for online available products that contain some forms of nanotechnology. Available at: http://www.nanotechproject.org/inventories/consumer/analysis_draft/ (Accessed 10 August 2008). Xia, T., M. Kovochich, M. Liong, J. I. Zink, and A. E. Nel (2008). Cationic polystyrene nanosphere toxicity depends on cell-specific endocytic and mitochondrial injury pathways. ACS Nano 2:85-96. Xiang, S. D., A. Scholzen, G. Minigo, C. David, V. Apostolopoulos, P. L. Mottram, and M. Plebanski (2006). Pathogen recognition and development of particulate vaccines: Does size matter? Methods 40:1-9. Yang, H., H. Li, and X. Jiang (2008). Detection of foodborne pathogens using bioconjugated nanomaterials. Microfluidics and Nanofluidics:1-13. Yang, S. C., and S. Benita (2000). Enhanced absorption and drug targeting by positively charged submicron emulsions. Drug Development Research 50:476-486. Yuan, Y., Y. Gao, J. Zhao, and L. Mao (2008). Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Research International 41:61-68.