Nuclear Medicine Instrumentation
advertisement

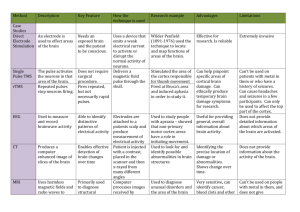
Nuclear Medicine Instrumentation 1. Medical background The unprecedented development in many areas of medicine during the last 50 years is to a large degree due to the availability of new diagnostic instruments. From the beginning of medical imaging in 1895 when Roentgen took the first X-ray picture, many methods and techniques have been developed, which were adapted or inspired from nuclear and particle physics instrumentation. A large part of medical imaging techniques concerns the viewing of anatomical structures of the body. X-ray computed tomography (CT) and magnetic resonance imaging (MRI) are sophisticated techniques of this type which yield high resolution images of physical structure parameters. However, in medical research and in the diagnosis of many medical conditions it is often necessary to use functional information. It is thus highly desirable to be able to acquire images of physiologic function to complement images of the anatomy. Certain biologically active pharmaceuticals which concentrate in different organs of the human body, when these are chemically labeled with specific radioactive materials and administered within to a patient, the distribution and functional information can be deduced from the gamma ray emission pattern. This class of imaging is known as nuclear medicine imaging. Nuclear medicine techniques are applicable in the diagnosis of a wide variety of diseases, they can be used for tumor detection, imaging of abnormal body functions and to quantitatively measure the heart or the brain function, for example since blood flow in the brain is tightly coupled to local brain metabolism and energy use, the 99mTc-HMPAO (hexamethylpropylene amine oxime) tracer can be used to assess brain metabolism regionally, in an attempt to diagnose and differentiate the different causal pathologies of dementia. Depending on the method of the radio pharmaceuticals labeling, nuclear medicine techniques can be split into: gamma or positron emission methods 2. Gamma Camera The first gamma camera was developed by Hal Anger in 1957, and his original design is still widely used today. This device gives projective images, when imaging with a gamma camera the patient is injected with a small amount of a radioactive imaging agent chosen to accumulate in specific regions. The radioisotope disintegrates by emitting a single gamma photon with an energy typically between 80 and 350 keV. 99mTc (140keV) is the most frequently used. The components making up the Gamma camera are the collimator, Scintillator, front end electronic and data acquisition system (Figure 1. 1.). NaI(Tl) Scintillator Crystal Collimator Light guide Phototomultiplier Tube Figure 1. 1. Conventional gamma camera The collimator is made of gamma ray absorbing material (Lead or Tungsten), which acts to select a given direction of photons incident to the camera. In parallel hole collimator only photons traveling in a direction parallel to the collimator holes will reach the scintillator detector. The collimator defines the geometrical field of view of the camera and determines both the spatial resolution and sensitivity of the system. After the collimator the gamma-rays are detected by a large single thallium-activated sodium iodide NaI(Ti)) scintillator crystal, typically about 50 cm in diameter. The interaction of the a gammaray with the crystal results in scintillation photons emitted isotropically. The emitted photons are detected by array of photomultiplier tubes (PMTs) which are optically coupled to the surface of the crystal detector. A PMT consist of two elements, a photo cathode coupled to an electron multiplier, the photo cathodes ejects electrons when stimulated by light photons. Electron multiplier consists of an arrangement of dynodes that serves both as efficient collection geometry for the photoelectrons and a amplifier, that greatly increases the number of electrons. After amplification, a typical scintillation pulse will give rise to 107-1010 electrons, sufficient to generate a strong charge signal that can be collected at the anode. At the photomultiplier output a amplifier, filters, shaper and line-driver are used to adapt and transport the pulse to the data acquisition system were the two coordinates of the interaction position are extracted from the amplitude distribution of the PMT signals and the total energy is obtained from their sum. The total sum allows discrimination between different isotopes or between scattered and direct photons. The data are then sent to a computer to process it into a readable image of spatial distribution of the organ activity. 3. Computed Tomography Computed tomography (CT) is a medical imaging method where digital geometry processing is used to generate a three-dimensional image slices of an object from a large set of two-dimensional images (projections) taken around a single axis of rotation. The mathematical principles of tomographic reconstruction have been known for a long time, but tomographic imaging techniques had to await the digital revolution before being implemented due to the computing power needed. The first practical application was made in 1960’s. Then in the 1970’s there was an explosion of activity with several techniques being developed simultaneously, most notably positron emission tomography (PET) and Xray computed tomography (CT). CT was invented in 1972 by Godfrey Hounsfield and Allan Cormack and they were later (1979) awarded the Nobel Prize for their contributions to medicine and science. The computed tomography technique has applications in non-medical fields as well, for example in astronomy where images taken at different times from different angles are combined and a more accurate composite image is constructed. The back projection algorithm was the first algorithm used for the image reconstruction, but because it produces blurred images projected data needs to be filtered before reconstruction. Depends on the application different filters can be applied. Filtered back projection algorithm is one of the most used reconstruction methods. 4. Single photon emission computed tomography (SPECT) SPECT imaging is performed by rotating a gamma camera around the object (or patient) and acquiring projections from multiple angles (Fig.1.2.). Figure 1. 2. A standard SPECT by rotating a single head gamma camera A topographic reconstruction algorithm is then applied to the 2D projections to reconstruct a 3D image, which can be used to show thin slices of the position and concentration of radionuclide distribution along a chosen axis. The use of the collimator results in low sensitivity, in order to increase the sensitivity and hence the scanning time, the SPECT are now being equipped with multi-detector systems for total-body, brain and heart scanning. The systems with 2 or 3 Anger type gamma cameras 180o, 120o or 90o apart with small or large filed of view are most popular. These systems have considerably improved the sensitivity, resolution and the scanning time comparing to the single camera systems. The parallel collimator can be replaced by pinhole collimator which allows geometric enlargements and obtain high resolution specialty for imaging small objects. The variable attenuation by the body of the photons emitted from the organ of interests and the incorrect counting of scattered photons in detector crystal can lead to significant underestimation of activity inside the body, compared to the surface and hence an image degradation. This problem is solved by incorporating a map of attenuation coefficients from transmission scan by X-ray CT into the reconstruction algorithm. Iterative reconstructions algorithms [1] are alternative algorithms which are growing in importance are used to reduce the image degradation. Despite being efficient and widely used, SPECT with the conventional gamma camera suffer from some limitations such us the non-uniformity, image distortion and degradation of the position towards the edge of the camera, it is bulky and heavy. The trend is towards SPECT systems based on compact and Dedicated gamma cameras for specific clinical applications and for small animal (molecular imaging). The proposed devices use new semiconductor detectors (Ge, CdTe or CdZnTe) where the gamma rays are converted directly to digital electronic signals, continuous or pixellated scintillator crystals coupled to an array of solid state photodiodes or to a Position Sensitive Photo-Multiplier Tube. 5. Positron emission tomography (PET) Positron emission tomography use biologically active tracers labeled with a position-emitting isotope. Following the decay, the emitted positron slows down and annihilates with electrons in tissue producing two back to back 511-keV photons. The detection of these two photons in coincidence defines a line along which the emission point is known to be located. By accumulating many annihilation events, the spatial distribution of the isotope, and therefore of the labeled radiopharmaceutical is reconstructed. The electronic coincidence detection of the two collinear 511 keV photons limits the position of the source to a line, there is no need of the collimator which is one of the great advantage of PET. Figure1. 3. is a schematic of the principle of the PET technique. Figure 1. 3. A schematic of the P ET principle 5.1. PET detectors and detector configurations 5.1.1. Detector materials There are three important practical features affecting the PET detector performances, the attenuation coefficient for the 511-keV photon, the light output, and the speed (decay time of the scintillator). The attenuation coefficient determines the detector sensitivity and it should be high, the energy resolution is improved with the high light output and a fast crystal leads to high count rate and narrow coincidence window and thus less random count rate. The choice of the detector depends on the application and the cost. The NaI(Tl) were used in early PET detectors, it has high light output and it is cheap, but it is stopping power is suboptimal compared to other crystals. Due it is greatest attenuation coefficient and it is cost BGO is widely used in current commercial PET systems. The disadvantages of BGO compared to other crystals include its low light output compared to NaI(Tl), high decay time compared to LSO, and it is emission at 500nm where PMTs are less sensitive. LSO is the most suitable crystal for today PET scanners; it has short decay time, high light lead, and high density. Table 1 resumes the characteristics of the crystals used for PET. 5.1.2. Detector configurations Different detector configurations have been developed for commercial PET scanners. The simple configuration is where the detectors are mounted on a rotating gantry (Fig. 6. a). For high sensitivity cameras the detectors are mounted on circular or polygonal rings. (Fig. 6. b) and (Fig. 6. c). modern PET systems have multiple rings configuration. Such systems have high performance at the expense of increased cost. Scintillator crystals PMT A Y PMT C PMT D X PMT B Figure1.1. 4 Block detector configuration for PET Conventional detectors consists of single scintillator coupled to a single PMT,today the most common configuration used for commercial scanners and small animal PET is the block detector configuration. Block detector consists of 2D array of single crystals from a cut of large cubic crystal and the cuts are filled with reflective material to optically isolate the single crystals from each other. The array is coupled to 4 PMTs with light sharing. State-of-art PET are using PSMT for more accurate positional and energy information. Solid state detectors offer high segmentation, hence higher spatial resolution. They are promising candidates for future PET generations. 5.2. Two dimensional (2D) and three dimensional (3D) PET scanners can be designed to operate in two dimensional (2D) or 3D mode. In 2D mode thin septa of lead or tungsten separate each crystal ring and coincidences are only recorded between detectors within the same ring or lying in closely neighboring rings (Fig. 7.a). This reduces the contributions from scatter and random coincidences with consequent reduction in the overall sensitivity. In 3D mode, the septa are removed, and coincidences are recorded between detectors lying in any ring combination (Fig. 7.b). This results in a fully 3D image and in substantial increase in the sensitivity, at the expense of an increased scatter fraction and count rate performance. a b Figure 1.1. 5 PET acquisition mode a) 2D with septa b) 3D without septa 5.3. Types of coincidence events Coincidence events in PET can be classified as: true, scattered and random (Fig. 8).True coincidences occur when both photons from an annihilation event are detected by detectors in coincidence, neither photon undergoes any form of interaction prior to detection, and no other event is detected within the coincidence time-window. A scattered coincidence is when at least one of the detected photons has undergone Compton scattering event prior to detection and because the direction of the photon is changed the event will be assigned to wrong line of response. Scattered coincidences add a background to the true coincidence distribution and causing the isotope concentrations to be overestimated. Random coincidences occur when two photons not arising from the same annihilation event are incident on the detectors within the coincidence time window, the rate of random coincidences increase roughly with the square of the activity in the field of view (FOV). Random coincidences add statistical noise to the data. The number of scattered and random events detected depends on the volume and attenuation characteristics of the object being imaged, and on the geometry of the camera. Scattered event True event Random event Figure 1.1. 6. Types of coincidence events in PET 6. New detectors for PET and SPECT 6.1. Scintillators Inorganic scintillator crystals are the most commonly used detectors for Gamma camera. The gamma photon interact within scintillator through Rayleigh, Compton, and photoelectric effects. The photon deposit it is energy at one location by photoelectric effect or at different position by Compton interaction. The absorbed energy causes the crystal to make a transition to higher energy state, from which it may undergo decay after a characteristic time by emitting lower energy photons that are detected by photodetector. Photoelectric and Compton cross-sections are a function of the density (ρ) and the effective atomic number (Zeff) of the crystal. A high density favors the interaction of the photon in the crystal, whilst a higher Zeff value increases the number of photoelectric occurrences with respect to Compton scattering. Therefore, high Zeff crystals are to be referred. The light yield and the decay time are important physical properties of the crystal. A high light output (number of photons per Mev) implies a better energy resolution, hence high positioning accuracy. Short decay time leads to high counting rat. The scintillation photon wavelength has to much the properties of the photodetectors. NaI(Tl) is the scintillator of choice for imaging with gamma camera due to it is light output 41000(Ph/Mev), which allows energy resolution of the order 9-11%(FWHM) at 140 keV. The drawback of NaI(T)l is that it cannot be produced in shape of long and thin pillars that can be arranged in segmented scintillator array due to its hygroscopicity. CsI(Tl) and YAP:Ce are suitable crystals for these geometries (Fig.2). In Table 1 we list the characteristic of most of the crystals available for gamma detection. Scintillator material Density (g/cm 3) Zeff Attenuation lenght for 511-Kev gammas (mm) Light output (ph/Mev) Decay time (ns) Emission wave length (nm) Refractive index BGO 7.1 75 10.4 9000 300 480 2.15 LSO 7.4 66 11.4 30000 40 420 1.82 NaI(Tl) 3.67 51 29.1 41000 230 410 1.85 CsI(Tl) 4.51 52 22.9 66000 900 550 1.80 GSO 6.7 59 14.1 8000 60 440 1.85 LuAP 8.3 64.9 10.5 12000 18 365 1.94 YAP 5.5 33.5 21.3 17000 30 350 1.94 Table 1. 1. Physical properties of scintillator materials commonly used for SPECT and PET 6.2. Photodetectors 6.2.1. Position-sensitive photomultiplier (PSMT) A PSPMT is position sensitive which means that photons impinging at different sites on the photocathode would give rise to pulses of varying amplitudes at the anodes outputs. Typical The output signal is read using independent multiple anodes where each anode is connected to an individual independent electronic chain or by means of current or charge-dividing center-of gravity where the anodes are connected through a resistive network and position signals are read from the two ends for both X and Y direction. For each event, time of interaction is provided by the last dynode. PSPMT coupled to a thin scintillator such as CsI(Tl) or NaI(Tl) are an attractive approach achieving good spatial and energy resolution. 6.2.2. Silicon photodiodes Photodiodes are semiconductor devices with a pn junction or pin structure where light absorbed in a depletion region generates electrical carriers and thus a photocurrent. Such devices can be very compact, fast, highly linear, and exhibit a high quantum efficiency. The most used for gamma ray imaging is the avalanche photodiode. Avalanche photodiode is a semiconductor-based photodiode which is operated with a relatively high reverse voltage (typically tens or even hundreds of volts), sometimes just below breakdown. In this regime, carriers (electrons and holes) excited by absorbed photons quickly get accelerated in the strong internal electric field, so that they can generate secondary carriers, as it is known in photomultipliers. The advantage of APDs is the high siagnal to noise ration due to high gain, and the faster response relative to PMTs and Photodiodes . APDs can be produced in arrays and used for very compact systems. 6.2.3. Semiconductor Detectors Semiconductor detectors are promising alternative to scintillators for gamma ray imaging due to their superiority for energy resolution, and they can be easily pixelated and read out directly. The most attractive semiconductor material is cadmium zinc telluride (CdZnTe). CdZnTe has very good absorption characteristics due to its high density(5.8 g/cm3) and effective atomic number (50), the attenuation length of 140 keV gamma-rays is being only 3 mm the photofraction is 81% , the absorption of a 140 keV produces approximately 3*104 which is a good charge yield , hence an excellent energy resolution . Due to the wide band gap it has a high resistivity which results in low noise characteristic. 7. Compton cameras In Compton camera the kinematics of Compton scatter is used as an electronic collimator to reconstruct the radioisotope distribution. The basic concept of Compton camera consists of two detectors scatter and absorber separated by a known distance, both with very good energy and spatial resolution. when gamma photon inter the camera , it interacts with the first detector and scatter to the second detector (Figure 1. 6). Detector 1 Detector 2 Figure 1.4.4: Principal of Compton camera By using the two detectors in coincidence, the total energy and the direction of travel from the first detector to the second can be extracted for each event. This information in conjunction with the Compton equation (bellow) can be used to back project a cone of all possible direction of the incident gamma ray. 1 1 cos 1 mo c 2 E E E re where is the cone semi-aperture and the Compton scatter angle; Ere is the recoil electron energy in the front detector; E is the source energy; and m oc2 is the rest mass of an electron (511keV). A large number of scattering events from a point source of gamma rays will define multiple cones which intersect at the location of the source (Figure 1.4.2). Figure 1.4.2: Cones intersection determine the interaction point 8. SPECT versus PET The use of the collimator in SPECT results in tremendous decrease in the resolution and the efficiency compared to PET where collimation is performed electronically which leads to high sensitivity. High sensitivity improve signal to noise ratio which correspond to improvement in image. With PET the whole scan is performed in short time compared to SPECT and multiple field of view can be scanned. Although SPECT imaging resolution is many times less than PET, the high cost of PET scanners, the need of the accelerator close the examination place, he availability of low cost SPECT pharmaceutical and the practical and economic aspects of SPECT instrumentation makes this technique of functional imaging attractive for a lot of clinical studies specially for the brain and the heart.