STGCL.SWP.36.1_BCA Protein Assay_April
advertisement

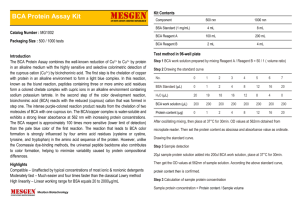
OHS026 Safe Work Procedure Faculty/Division Medicine School/ Divisional Unit St George Clinical School Document number Initial Issue date Current version STGCL.SWP.36.1 10/04/2010 1.0 Current Version Issue date 10/04/2010 Next review date 10/04/2012 The Writing Safe Work Procedures Guideline (OHS027) should be consulted to assist in the completion of this form. Safe Work Procedure Title and basic description Title: : Bicinchoninic Acid (BCA) Protein Assay (96 well plate format) Description: This Safe Work Procedure describes how to perform the BCA Protein Assay (96 well plate format) safely Associated risk assessment title and location: STGCL.RA.36.1 Level 3 Research and Education Center, St George Clinical School Describe the activity or process This SWP covers the protocol for determining protein concentrations from cell lysates using the BCA (bicinchoninic acid) reagents from Pierce Biotechnology. Read all relevant MSDSs and the attached Risk Assessment before proceeding with this protocol. Note that the BCA Assay is appropriate for determining the protein concentration of cell lysates and other protein extracts, but not for tissue culture supernatants (conditioned medium, etc.), as the phenol red in the medium can produce a non-specific reaction. Procedure: 1. Preparation of albumin standards: i. The albumin standard is provided as 2 mg/mL stock in a glass ampoule. Snap the top of the ampoule at the marked line and then transfer the stock solution to a 1.5 mL Eppendorf tube. (NB: breaking the ampoule is a cutting risk) ii. Next, using water as a diluent, perform serial two-fold dilutions of the 2 mg/mL stock solution to obtain the following concentrations: 1, 0.5, 0.25, and 0.125 mg/mL. Use water alone for 0 mg/ml. iii. Store these solutions at 4oC when not in use. 2. Into individual wells of a non-sterile 96-well plate pipette the following: i. 10 µL of each albumin standard in triplicate (2 mg/mL to 0 mg/mL) ii. 10 µL of each buffer used to prepare the lysate(s) in triplicate (e.g. M-PER) iii. 10 µL of each lysate sample in triplicate (if you suspect your sample may be too concentrated, dilute it first) 3. To prepare the BCA working solution (NB: both assay components are hazardous substances), mix the two reagents such that Reagents A and B are in a 50:1 ratio. First, add up the total number of test wells and add a few more to allow for pipetting error; multiply this number by 200 µL to give the volume of Reagent A required. Divide this figure by 50; this is the volume of Reagent B required. Add these two volumes together in a 15 or 50 mL tube, and mix by inversion. ___________________________________________________________________________________________________________ ___________ Page 1 of 3 Safe Work Procedure Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 1.2, 15/08/2007 Describe the activity or process 4. To each test well, add 200 µL of the BCA working solution, taking care not to touch the protein test samples whilst pipetting. 5. Cover the plate with foil and incubate for 30 minutes at 37oC. If the samples are very concentrated, you may incubate for less time than this, but be aware that this may interfere with the development of the standard curve (it is better to dilute your samples, repeating the assay if necessary) 6. The plate is read at 570 nm following a pre-shaking step of 5 seconds (inside, low speed). 7. Convert the data to Excel and save it; dispose of the plate as cytotoxic waste. Analyse the data using the albumin standards to generate a standard curve, and the buffer only test wells as the blanks. List all resources required including plant, chemicals, personal protective clothing and equipment, etc BCA Protein Assay Reagent A (Pierce, cat. # 23223) (NB: this is a hazardous substance) BCA Protein Assay Reagent B (Pierce, cat. # 23224) (NB: this is a hazardous substance) Albumin Standard (2 mg/mL) (Pierce, cat. # 23209) Non-sterile 96-well plate(s) (Greiner, pack of 5, cat. # 655 101) Plate reader, 570 nm filter PPE (gloves, gown, goggles) List potential hazards and risk controls including specific precautions required List emergency shutdown instructions List clean up and waste disposal requirements ___________________________________________________________________________________________________________ ___________ Page 2 of 3 Safe Work Procedure Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 1.2, 15/08/2007 List legislation, standards and codes of practice used in the development of the SWP MSDS for Pierce BCA Assay Supervisory approval, training, and review Supervisor: Prof S. Krilis (Immunology) Signature: Supervisor: Signature: Supervisor: Signature: Supervisor: Signature: Plant custodian: Prof S. Krilis Signature List competency required – qualifications, certificates, licencing, training - eg course or instruction: N/A SWP review date: 10th April 2012 Responsibility for SWP review: Gao Lu ___________________________________________________________________________________________________________ ___________ Page 3 of 3 Safe Work Procedure Date Effective: 01/01/2007 Uncontrolled document when printed Current Version: 1.2, 15/08/2007