Prediction of dry matter and crude protein content in fresh grass
advertisement

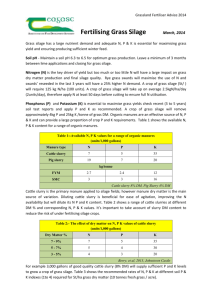
1 Prediction of Dry Matter and Crude Protein Content in Fresh Grass Silage by Near Infrared Spectroscopy M. Vranić*, M. Knežević*, Zs.Seregély**, K. Bošnjak*, J. Leto*, G. Perčulija* *Department of Crop, Forage and Grass Production, Faculty of Agriculture, The University of Zagreb, Croatia ** Corvinus University of Budapest, H1118 Budapest, Ménesi út 45., Hungary Introduction Grass silage varies greatly in terms of chemical and biological composition due to the impact of factors such maturity stage at harvesting, sward botanical composition, level of fertilisation, climate and ensiling techniques on the fermentation process in the silo. The feeding value of silage for animal production depends on the concentration of nutrients and the voluntary intake (Gordon and Murdoch, 1978), which are mainly affected by digestibility, and DM concentration (Steen et al., 1998). Highly digestible silage alone provides nutrients for maintenance and the production of only 14.3 kg of milk per day (Castle et al., 1980). This is the reason the silage is rarely used as the sole food for highly yielding dairy cows and has to be supplemented with other feeds, normally concentrates, that are high in energy (McKee et al., 1996). Supplementation represents a substantial part of the variable on-farm costs. Analytical procedures are slow and expensive while the rapid analysis of fresh grass silage samples (a very heterogeneous, high moisture forage) on DM and CP would reduce the cost of analysis and provide essential data for rationing purposes. It has been shown (Steen et al., 1995) that NIRS can accurately predict the voluntary intake of grass silage by cattle. It also has the potential to enhance understanding of grass silage digestion and cell wall degradation (Wilman et.al., 2000), and it is the most precise approach available among laboratory methods to predict in vivo organic matter digestibility of grass silage (Barber et.al., 1990). There are advantages in analysing fermented forages in their natural state due to avoidance of analytical errors caused by the loses of volatile compounds by oven-drying. The objective of this study was to determine the accuracy of near infrared reflectance spectroscopy (NIRS) for measuring DM (g kg-1) and CP (gkg-1DM and %) contents of fresh grass silage samples. 2 Materials and methods The silages were harvested at five different maturity stages in 2002 from the leafy to the late blooming stage at 6-10 days intervals. The sward was fertilised with 36 kg ha-1 nitrogen (N), 117 kg ha-1 phosphorus (P2O5) and 117 kg ha-1 potassium (K2O) (NPK 8:26:26) in early spring. Thirty-five days prior to harvesting 40.5 kg ha-1 N was applied in the form of calcium ammonium nitrate (CAN) (150 kg ha-1). Green and dry matter yield (t ha-1) was determined at mowing by calculating the weight of forage taken by a square frame (0.25x0.25m). Botanical composition was determined from the same samples by manual separation of sward components (grass, clover, other forage plants). The herbage was wilted for 8 hours before baling with a round baler. Bales were ensiled on each maturity date (wrapped in 4 layers of 500 mm-wide white plastic film). After 60 days, all bales were cored through twice using a motorised sampler fitted with a 50-mm diameter, 500 mm long steel tube. The cored material from each bale was then bulked, mixed and subsampled prior to scanning. Without any pre-treatment, three independent scans were recorded from each of 103 samples, using an NIRSystem Model 6500 spectrometer system (Foss-NIRsystem, Sweden) fitted with a sample transport module and a natural product sample cup (56 x 240 mm). Samples were scanned (32 scans co-aded) using the ISI SCAN Version 1.0 (Infrasoft International, Port Matilda, PA, USA) from 1100 to 2498 nm in reflectance mode (R mode: PbS detector). Data were collected every 2 nm (700 data points per spectrum). The mean spectral value of each sample was calculated using the WIN ISI III Version 1.5. After scanning the the fresh samples, the DM contents of the same samples was determined immediately by oven drying to a constant weight at 60°C in a forced-draught oven. The samples were dried and ground to pass a 1mm screen. Total nitrogen (TN) concentrations were determined using a Gerhardt nitrogen analyser and expressed as crude protein (CP g kg-1 DM) (TN x 6.25) and percent on a fresh basis. Data were analysed using mixed model procedures (SAS, 1999). Mean separation was calculated using the least significant difference (LSD) values if the F-test was significant at P=0.05. In performing measurements with the NIRSystem Model 6500 spectrometer, hundreds of data are generated for each sample and it is obvious that some data reduction method is needed to facilitate data interpretation. The user is interested in the compositional data of the samples rather than in the spectral data. Thus, there is a need for data processing methods that transform the measured spectral data into the sample properties (concentration of the constituents) of interest. For producing such models that determine the equations describing the relationship between spectral data and constituent values, three different methods (MLR, PCR and PLS) were used. MLR uses a multiterm linear polynomial to describe this relationship using only several spectral data measured as “characteristic” wavelengths. With OCA, all spectral data are used, reducing the dimensionality of the data set by looking for orthogonal directions in spectral data space along which the variance of the data set is maximised. These directions are called principal components. Thus, the first principal component (PC1) is determined as the direction in spectral data space that corresponds to the 3 largest variation in the data set. The second principal component (PC2) is calculated as the direction perpendicular to Pc1 along which the remaining variation in the data set is the largest. This procedure is repeated until no variation is left in the data set. PLS uses not only the spectral information in the data set but also incorporates information about sample properties, e.g. concentrations to determine the most useful orthogonal directions in spectral space. Results The sward consisted of 80.6% grass, 11.69% clover and 7.71% of other forage. The yields of herbage green mass (t ha-1) were 32.18; 32.52; 29.91; 25.79 and 24.36 from early to late cut respectively. The yields of herbage dry matter (t ha-1) were 4.842; 5.429; 6.466; 7.026 and 7.255 from early to late cut respectively. The latest-cut silage had the highest DM concentration (P<0.05) compared with earlier-cut silage. Because the DM concentration in the silage was high (Table 1), polluting effluent production from the silage was not observed. Table 1. Dry matter and crude protein content of samples as determined by laboratory reference methods Cut n DM g kg-1 min mean 1 2 3 4 5 Total 22 22 25 19 15 103 bc 472.60 439.14 cd 432.36 d 481.11 b 540.93a 467.21 345 326 368 216 280 216 max 597 504 520 560 629 629 mean a 6.05 5.29 b 4.40 cd 4.02 d 4.79 c 4.93 CP % min 4.48 3.62 3.42 1.88 2.74 1.88 max 7.50 6.90 5.12 4.4 6.06 7.50 CP g kg-1 DM min mean a 128.40 120.49 b 102.50 c 83.85 d 88.99 d 106.47 100.1 108.6 86.14 76.22 72.40 72.40 max 150.1 151.8 116.6 89.72 103.4 151.8 Means with a different letters within columns are significantly different (P<0.05). As anticipated, the concentration of crude protein (CP) declined from the early to fourth-cut silage, but the latest cut showed higher CP content (P<0.05) than the fourth-cut silage (Table 1). For quantitative evaluation methods multiple linear regression (MLR), principal component regression (PCR) and partial least squares regression (PLS) were used to for data modelling and for predicting the investigated chemical constituents. For model validation, full crossvalidation was used; each case was predicted by the model derived from all other remaining spectra. The results of full cross-validation is the average of the standard error of prediction values produced during each cross-validation step. The recorded log (1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) before the analysis. Optimum models were achieved using 9 orthogonal factors with the PLS method and 5 PCs with PCR, whilst for MLR only the spectral values measured at two wavelengths were used. 4 Figure 1, 4, and 7 show the relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for dry matter (g/kg fresh sample) for the 103 fresh silage samples investigated. These were determined using PLS, PCR, and MLR methods, respectively, for evaluation. The results of the calibration procedures are summarised in the upper figure, while the cross-validated results are presented in the lower figures. It can be seen in the figures that the smallest value for prediction error (SEP = 29,7 dry matter g/kg fresh sample) with the highest multiple correlation coefficient (r = 0,91) was provided by the PLS method. The application of PCR using the whole wavelength region (1100-2500 nm, 700 data) requiring scanning type spectrometers as with PLS, resulted in an almost twice as high prediction error. The same order of magnitude could be obtained for SEP value by using MLR. Compared to PCR and PLS the MLR model uses only two characteristic wavelengths for the calculation, creating the conditions for constructing cheaper single-purpose filter instruments (In reality – using second derivative – 6 spectral data point are needed). Similarly as in figures 1, 4, and 7, the relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for crude protein (% FW) of 103 fresh silage samples, determined by the above three methods are shown in Figures 2, 5 and 8, while for crude protein content (g/kg DM) the results are shown in Figures 3, 6 and 9. By studying the figures presented, the same conclusion can be drawn as in case of the investigation of dry matter content discussed in the previous paragraph. PLS provided the highest correlations and the smallest prediction errors. 5 Computed Value (NIR data) 800 600 dry matter (g/kg fresh sample) Number of elements: 103 Standard error of calibration: 22,2 Multiple correlation coefficient: 0,95 400 200 200 400 500 Constituent (LAB data) 600 700 400 500 Constituent (LAB data) 600 700 300 800 Computed Value (NIR data) dry matter (g/kg fresh sample) 600 Number of samples: 103 Standard error of prediction: 29,7 Multiple correlation coefficient: 0,91 400 200 200 300 Figure 1 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for dry matter (g/kg fresh sample) of 103 fresh silage samples, determined using PLS method for evaluation. Calibration results above, cross-validated results below. Wavelength range: 1100-2500 nm. The log(1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 6 Computed Value (NIR data) 8 6 crude protein % Number of elements: 103 Standard error of calibration: 0,24 Multiple correlation coefficient: 0,97 4 2 2 3 4 6 5 Constituent (LAB data) 7 8 7 8 8 Computed Value (NIR data) crude protein % 6 Number of samples: 103 Standard error of prediction: 0,31 Multiple correlation coefficient: 0,95 4 2 2 3 4 5 Constituent (LAB data) 6 Figure 2 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for crude protein % of 103 fresh silage samples, determined using PLS method for evaluation. Calibration results above, cross-validated results below. Wavelength range: 1100-2500 nm. The log(1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 7 Computed Value (NIR data) 150 120 crude protein content (g/kg DM) Number of elements: 103 Standard error of calibration: 5,86 Multiple correlation coefficient: 0,96 90 60 60 Computed Value (NIR data) 150 120 80 120 100 Constituent (LAB data) 140 160 120 100 Constituent (LAB data) 140 160 crude protein content (g/kg DM) Number of elements: 103 Standard error of prediction: 6,65 Multiple correlation coefficient: 0,94 90 60 60 80 Figure 3 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for crude protein content (g/kg DM) of 103 fresh silage samples, determined using PLS method for evaluation. Calibration results above, cross-validated results below. Wavelength range: 1100-2500 nm. The log(1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 8 800 Computed Value (NIR data) dry matter (g/kg fresh sample) 600 Number of elements: 103 Standard error of calibration: 50,4 Multiple correlation coefficient: 0,69 400 200 200 300 400 500 Constituent (LAB data) 600 700 600 700 800 Computed Value (NIR data) dry matter (g/kg fresh sample) 600 Number of samples: 103 Standard error of prediction: 51,7 Multiple correlation coefficient: 0,66 400 200 200 300 400 500 Constituent (LAB data) Figure 4 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for dry matter (g/kg fresh sample) of 103 fresh silage samples, determined using PCR method for evaluation. Calibration results above, cross-validated results below. Wavelength range: 1100-2500 nm. The log(1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 9 Computed Value (NIR data) 8 6 crude protein % Number of elements: 103 Standard error of calibration: 0,60 Multiple correlation coefficient: 0,79 4 2 2 3 4 5 6 7 8 6 7 8 Constituent (LAB data) Computed Value (NIR data) 8 6 crude protein % Number of elements: 103 Standard error of prediction: 0,64 Multiple correlation coefficient: 0,76 4 2 2 3 4 5 Constituent (LAB data) Figure 5 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for crude protein % of 103 fresh silage samples, determined using PCR method for evaluation. Calibration results above, cross-validated results below. Wavelength range: 1100-2500 nm. The log(1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 10 Computed Value (NIR data) 150 120 crude protein content (g/kg DM) Number of elements: 103 Standard error of calibration: 7,94 Multiple correlation coefficient: 0,91 90 60 60 Computed Value (NIR data) 150 120 80 120 100 Constituent (LAB data) 140 160 120 100 Constituent (LAB data) 140 160 crude protein content (g/kg DM) Number of elements: 103 Standard error of prediction: 8,99 Multiple correlation coefficient: 0,89 90 60 60 80 Figure 6 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for crude protein content (g/kg DM) of 103 fresh silage samples, determined using PCR method for evaluation. Calibration results above, cross-validated results below. Wavelength range: 1100-2500 nm. The log(1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 11 Calibration (dry matter g/kg) The standard error of calibration = 47,4 The multiple correlation coefficient = 0,73 Computed Value (NIR data) dry matter (g/kg fresh sample) Number of elements: 103 Constituent (LAB data) Cross Validation (dry matter g/kg) The standard error by cross validation = 48,4 The multiple correlation coefficient = 0,71 Figure 7 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for dry matter (g/kg fresh sample) of 103 fresh silage samples determined using MLR method for two wavelengths. Calibration results above, crossvalidated results below. Wavelength range used for selecting the two characteristic wavelengths: 1100-2500 nm. The log(1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 12 Calibration (crude protein %) The used two wavelengths: 1948, 2166 nm The standard error of calibration = 0,58 The multiple correlation coefficient = 0,81 crude protein % 103 Computed Value (NIR data) Number of elements: Constituent (LAB data) Cross Validation (crude protein %) The standard error by cross validation = 0,59 The multiple correlation coefficient = 0,79 Figure 8 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for crude protein % of 103 fresh silage samples determined using MLR method for two wavelengths. Calibration results above, cross-validated results below. Wavelength range used for selecting the two characteristic wavelengths: 1100-2500 nm. The log(1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 13 Calibration (crude protein g/kg DM) The used two wavelengths: 1142, 2080 nm The standard error of calibration = 8,80 The multiple correlation coefficient = 0,89 Computed Value (NIR data) crude protein content (g/kg DM) Number of elements: 103 Constituent (LAB data) Cross Validation (crude protein g/kg DM) The standard error by cross validation = 8,96 The multiple correlation coefficient = 0,89 Figure 9 The relationship between chemical (LAB) data and data calculated from near infrared (NIR) spectral data for crude protein content (g/kg DM) of 103 fresh silage samples determined using MLR method for two wavelengths. Calibration results above, crossvalidated results below. Wavelength range used for selecting the two characteristic wavelengths: 1100-2500 nm. The log (1/R) spectra were smoothed (5 points) and transformed to second derivative (7 points) 14 Discussion Samples selected for calibration (Table 1) comprised a wide range from 216 to 629 g kg-1 for dry matter (DM), from 1.88 to 7.5 % for crude protein (CP) and from 72.4 to 151.8 for CP g kg-1DM. DM content increases and CP decreases with advanced maturity (Castle et al., 1980; Cushnahan et al., 1996). This is confirmed by a certain instance in our study as fifth cut silage shows the highest mean DM value, but the last cut silage had higher CP content compared with the third cut silage that was probably caused by higher ammonium concentration. PLS regression analysis of the spectral data with DM content resulted in the highest prediction correlation coefficients (R) compared with PCA and MLR method (Table 2). Also, differences in standard error of full cross-validation (SECV) for DM prediction show the SECV achieved by PLS (29.74) was more than 40% lower compared with PCR (51.64 g kg-1) or MLR methods (48.74 g kg-1). Table 2. Comparison of the accuracy of the LAB data with the results achieved by near infrared spectroscopy using three different methods for evaluation PLS PCR MLR LAB data R SEP R SEP R SEP SEL dry matter (g/kg 0,90 29,74 0,66 51,64 0,71 48,74 6.79 fresh sample) crude protein % 0,95 0,31 0,75 0,64 0,79 0,59 0.095 crude protein (g/kg DM) 0,94 6,64 0,89 8,99 0,89 8,96 1.91 The SECV values for CP of 6.64, 8.99 and 8.96 (on DM basis) for PLS, PCR and MLR, respectively, are comparable with Murray’s (1986) results that was 8.4 and with the data reported by Flinn and Murray (1987) being 10.5 and 11.1. The SECV for CP of 6.64; 8.99 and 8.96 g kg-1DM gained by PLS, PCR and MLR methods, respectively, is comparable with the range of 6.0-13.4 g kg-1 reported by Norris et al. (1976), Marten et al. (1983) and Murray (1986). The two wavelengths chosen for calibration by the MLR method were 1366 and 2418 nm for DM (g kg-1); 1142 and 2080 nm for CP (g kg-1DM), and 1948 and 2166 for CP (% FW). They are not within the range of 2100-2164 quoted by Redshaw et al. (1986) and Murray (1986). The MLR evaluation method for CP (gkg-1DM) indicates a strong relationship (R2 of 0.88) with spectroscopic data. Several authors (Windham and Flinn, 1992; Rosental and Williams, 1996; Blanco et al., 1998) compared results obtained by MLR and PLS in different products and concluded PLS and MLR give nearly the same prediction errors. In our study, PLS was more accurate than MLR method. One of the reasons might be that 9 components were used for PLS compared 15 with 5 PC’a used for PCR and 2 wavelengths used for MLR. In our paper the SEP values were all higher than SEC results, similar to the ones reported by Stimson et al. (1991). The lowest SEP achieved for CP (%) content among the methods applied may be due to the fact the samples were scanned fresh and CP content in % was recalculated to the fresh weight while DM (gkg-1DM) and CP (gkg-1DM) were expressed on DM basis. Differences between SEC and SEP values may be due to the limited number of samples. This clearly reflects the problem of obtaining representative samples in practice. Also, grass silage samples, which are very heterogeneous, were scanned fresh without any pre-treatment while for wet chemistry analysis the same samples were dried and ground. Although errors are often slightly higher for samples scanned in their natural state than for dried and milled samples, this is balanced by the ability to scan much larger samples, the avoidance of compositional losses and changes due to oven drying and a major reduction in analysis time and cost due to no sample preparation being necessary. The findings indicate spectroscopic data evaluated by PLS method were strongly related to reference values and had lower SECV values compared with PCA and MLR methods. CP (gkg-1) content in fresh grass silage samples determined by MLR method (R2=0.88) suggests that this trait may be also accurately estimated by inexpensive filter instruments. Conclusion The NIRS estimation of forage chemical composition is a relatively inexpensive, a rapid and a reliable method compared with reference methods. It requires a relatively small quantity of sample and predicts several concentrations of components simultaneously. An important advantage of NIRS is its ability to analyse samples without chemical treatment, hence costs and chemical wastes can be reduced by the use of accurate NIRS equation. The success of NIRS analysis depends almost entirely on the reliability of the primary, reference data, used at calibration. NIRS can predict DM and CP content in fresh grass silage samples without costly or lengthy pre-treatment as shown in this paper. Nevertheless, a satisfactory accuracy with an average standard error of prediction by PLS method of 29.74, 0.31 and 6.64 for DM, CP (%) and CP (gkg-1), respectively, was obtained. The comparison of validation statistics (R2 and SECV) among PLS, PCR and MLR equations (with two wavelengths per variable) showed PLS to be the most accurate. (However, it does use 9 factors compared with 5 for PCA and 2 for MLR.) The MLR evaluation method for CP (gkg-1DM) has the potential to be used for industry requirements, as it needs less sophisticated and cheaper instrumentation using only a few wavelengths. Acknowledgments The authors express their sincere thanks to Professor Ian Murray from SAC for introducing them to the NIR technology and Professor Károly Kaffka from Corvinus University of Budapest, Hungary for providing helpful comments on the results. Thank also goes to Dr. Ian 16 Lane from ULG Consultants, UK for his technical assistance in establishing NIR unit within the Faculty of Agriculture, the University of Zagreb. References BARBER, G.D., GIVENS, D.I., KRIDIS, M.S., OFFER, N.W., MURRAY, I. (1990) Prediction of the organic matter digestibility of grass silage. Animal Feed Science and Technology, 28, pp 115-128. BLANCO, M., COELLO, J., ITURRIAGA, H., MASPOCH, S., GONZALEZ, R. (1998), Mikrochim. Acta 128, p. 235. CASTLE, M.E., RETTER, W.C., WATSON, J.N. (1980) Silage and milk production: a comparison between three grass silages of different digestibilities. Grass and Forage Science, 35, 219-225. CUSHNAHAN, A., MAYNE, C.S., GOODALL, E.A. (1996) Effects of stage of maturity and period of ensilage on the production and utilization of grass silage by dairy cows. In: Jones, D.I.H., Jones R., Dewhurst, R., Merry, R., Heigh, P.M. (eds) Proceedings of Eleventh International Silage Conference, IGER, Aberystwyth, 78-79. FLINN, P.C., MURRAY, I. (1987) Potential of near infrared reflectance spectroscopy (NIR) for evaluation of herbage quality in Southern Australia. In: Temperate Pastures, Their Production, Use and Management. Wheeler J.L., Pearson C.J. and Robards G.E. (eds.) Australia: CSIRO. GORDON, F.J., MURDOCH, J.C. (1978) An evaluation of a high-quality grass silage for milk production. Journal of the British Grassland Society, Volume 33, 5-11. MARTEN, G.C., HALGERSON, J.L., CHERNEY, J.H. (1983) Quality prediction of small grain forage by near infrared reflectance spectroscopy. Crop Science, 23, 94-96. MCKEE, C.A., CUSHNAHAN, A., MAYNE, C.S., UNSWORTH, E.F. (1996) The effect of partial replacement of grass silage with fresh grass on rumen fermentation characteristics and rumen outflow rates in cattle, 51, pp 32-41. MURRAY, I. (1986) Near infrared reflectance analysis of forages. In: Haresign W. and Cole D.J.A. (eds.). Recent Advances in Animal Nutrition. London: Butterworths. NORRIS, K.H., BARNES, R.F., MOORE, J.E., SHENK, J.S. (1976) Predicting forage quality by infrared reflectance spectroscopy. Journal of Animal Science, 43, 889’897. REDSHAW, E.S., MATHISON, G.W., MILLIGAN, L.P., WEISENBURGER, R.D. (1986) Near infrared reflectance spectroscopy for predicting forage composition and voluntary consumption and digestibility in cattle and sheep. Canadian Journal of Animal Science, 66, 103-115. ROSENTAL, B., WILLIAMS, P. (1996) The Future Waves. In: Near Infrared Spectroscopy. Ed.by A.M.C. Davies and P. Williams. NIR Publications, Chichester, UK, p.1. SAS (1999) SAS® Software, SAS Institute Inc., Cary, North Carolina, USA STEEN, R.W.J., GORDON, F.J., MAYNE, C.S., POOTS, R.E., KILPATRICK, D.J., UNSWORTH, E.F., BARNES, B.J., PORTER, M.G., PIPPARD, C.J. (1995) Prediction 17 of the intake of grass silage by cattle. Recent Advances in Animal Nutrition, Nottingham University Press (4, 67-89) STIMSON, C., KELLAWAY, R.C., TASSELL, R.J., ISON, R.L. (1991) Prediction of the nutrient content of botanical fractions from annual legumes by near infrared reflectance spectroscopy. Grass and Forage Science, 46, 99-105. STEEN, R.W.J., GORDON, F.J., DAWSON, L.E.R., PARK, R.S., MAYNE, C.S., AGNEW, R.E., KILPATRICK, D.J., PORTER, M.G. (1998) Factors affecting intake of grass silage by cattle and prediction of silage intake. Animal Science, 66, pp 115-128. WILMAN, D., FIELD, M., LISTER, S.J., GIVENS, D.I. (2000) The use of near infrared spectroscopy to investigate the composition of silages and the rate and extent of cell-wall degradation. Animal Feed Science and Technology, 88, pp 139-151. WINDHAM. W.R., FLINN, P.C. (1992) Bridging the Gap between Data Analysis and NIR Appliations. In: Near Infrared Spectroscopy, Ed.by K.I. Hildrum, T. Isaksson, T.Naes and A., Tandberg. Ellis Horwood, Chichester, UK, p. 459.