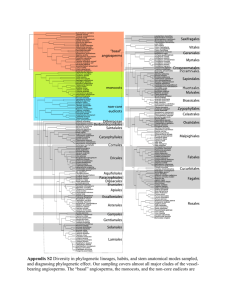
Our phylogeny was based on the most recent taxonomy by Frost
advertisement

SUPPORTING INFORMATION Global patterns of amphibian phylogenetic diversity Susanne A. Fritz and Carsten Rahbek Journal of Biogeography Appendix S1: Construction of the global amphibian phylogeny. Our phylogeny was compiled by hand based on a recent taxonomy by Frost (2009). To improve phylogenetic resolution above the genus level, we followed published molecular phylogenic studies. The family backbone followed Frost et al. (2006) with these updates: their Amphignathodontidae and Cryptobatrachidae were included in Hemiphractidae (Wiens et al., 2007a; Guayasamin et al., 2008), so the Nobleobatrachia consisted of the newly defined Hemiphractidae and Meridianura; the Meridianura consisted of Athesphatanura and Terrarana (Hedges et al., 2008), which corresponds to Frost et al.’s (2006) Brachycephalidae; family relationships within Terrarana followed Hedges et al. (2008); family relationships within Leptodactyliformes followed Grant et al. (2006). Family relationships within Natatanura were from different sources: Ptychadenidae were placed basally (Bossuyt et al., 2006; Frost et al., 2006; Van Bocxlaer et al., 2006; Wiens et al., 2009); Africanura and Aglaioanura were preserved as valid clades (Bossuyt et al., 2006; Van Bocxlaer et al., 2006); and the Victoranura constituted a polytomy of Africanura, Aglaioanura, Ceratobatrachidae, Micrixalidae, Nyctibatrachidae and Ranixalidae due to disagreement and low branch support values in the literature (Bossuyt et al., 2006; Van Bocxlaer et al., 2006; Wiens et al., 2009). Within families, we followed a range of published sources for between-genera relationships (Table S1). Species were added on as within-genera polytomies, so the species-level tree contains all currently recognized 6433 amphibian species (Frost, 2009). We assumed monophyly of genera, and included species listed in quotes in their assigned genus (these species are traditionally included in their assigned genus, but pending reclassification based on phylogenetic analysis): Bufo (Anura: Bufonidae) included the species arabicus, atukoralei, beddomii, brevirostris, dhufarensis, dodsoni, hololius, kotagamai, koynayensis, mauritanicus, olivaceus, parietalis, pentoni, scaber, scorteccii, 1 silentvalleyensis, stejnegeri, stomaticus, stuarti, sumatranus, tihamicus and valhallae; Colostethus (Anura: Dendrobatidae) included poecilonotus and ramirezi; Hyalinobatrachium (Anura: Centrolenidae) included eurygnathum, parvulum, and uranoscopum; Hyla (Anura: Hylidae) included antoniichoai, helenae, imitator, inframaculata and warreni. Exceptions to this rule followed published literature: ‘Ingerana’ baluensis (Anura, Dicroglossidae) was assigned its own genus in Ceratobatrachidae (Frost et al., 2006), and ‘Prostherapis’ dunni (Anura, Aromobatidae) was assigned to the genus Aromobates (Grant et al., 2006). A short version of this description has previously been published (Olalla-Tárraga et al., 2011), because that study used the phylogeny for some analyses published in their Appendix S3. Table S1 Sources of within-family phylogenetic relationships between amphibian genera. For families not in this table, we followed Frost et al. (2006), with genera missing from that study placed according to Frost (2009). If the position of genera was not known, they were added into a polytomy basal to their family. Family Reference Aromobatidae Grant et al. (2006) Brachycephalidae Hedges et al. (2008) Bufonidae Van Bocxlaer et al. (2010) Caeciliidae Zhang & Wake (2009), Wilkinson & Nussbaum (2006) Centrolenidae Guayasamin et al. (2008) Ceratobatrachidae Wiens et al. (2009) Ceratophryidae Grant et al. (2006) Craugastoridae Hedges et al. (2008) Cycloramphidae Grant et al. (2006) Dendrobatidae Grant et al. (2006) 2 Dicroglossidae Wiens et al. (2009), Bossuyt et al. (2006), Ohler & Dubois (2006) Eleutherodactylidae Hedges et al. (2008) Hemiphractidae Wiens et al. (2007a); corresponds to the families Amphignathodontidae, Cryptobatrachidae and Hemiphractidae of Frost et al. (2006) Hylidae Wiens et al. (2010), Smith et al. (2007), Faivovich et al. (2005) Hylodidae Grant et al. (2006) Hynobiidae Subfamily relationships according to Frost (2009); relationships within Hynobiinae followed Zhang et al. (2006) Ichthyophiidae Frost (2009) Leptodactylidae Grant et al. (2006) Leiuperidae Grant et al. (2006) Mantellidae Subfamily relationships according to Wiens et al. (2009); relationships within Mantellinae followed Glaw & Vences (2006) Megophryidae Delorme et al. (2006); relationships within Leptobrachiinae followed Fu & Bi (2007) Microhylidae Genera unassigned to subfamilies were included in a polytomy basal to the family; subfamily relationships mostly followed Van Bocxlaer et al. (2006), but also consider Frost et al. (2006) and the reanalysis of their data as well as original data in van der Meijden et al. (2007)1; relationships within Asterophryninae followed Köhler & Günther (2008)2; relationships within Cophylinae followed original data from van der Meijden et al. (2007) and Wollenberg et al. (2008)3; relationships within Gastrophryninae followed Frost et al. (2006) and the original data in van der Meijden et al. (2007); relationships within Microhylinae followed Van Bocxlaer et al. (2006), with missing taxa added according to van der Meijden et al. (2007) and Frost et al. (2006) Pipidae Frost et al. (2006), with Pseudhymenochirus added as the sister genus of Hymenochirus following Cannatella & Trueb (1988) 3 Plethodontidae Subfamily relationships were unresolved (Frost, 2009); relationships within Plethodontinae and Spelerpinae followed Vieites et al. (2007), within Bolitoglossinae they followed Wiens et al. (2007b) Ptychadenidae Wiens et al. (2009) Pyxicephalidae Wiens et al. (2009), Scott (2005) Ranidae Wiens et al. (2009), Stuart (2008), Che et al. (2007) Rhacophoridae Wiens et al. (2009), Li et al. (2008) Salamandridae Zhang et al. (2008) Strabomantidae Hedges et al. (2008) 1 Phrynomerinae was the sister group to all microhylids assigned to subfamily [Van Bocxlaer et al. (2006); reanalysis of Frost et al.’s (2006) data in van der Meijden et al. (2007)]; the sister clade to Phrynomerinae formed a polytomy, consisting of Otophryninae, Scaphiophryninae, Hoplophryninae, Gastrophryninae and an unnamed clade, because the published topologies differed widely and none recovered basal relationships with high support; the unnamed clade, which was supported by Van Boxclaer et al. (2006) and van der Meijden et al. (2007), consisted of two clades as follows: (Melanobatrachinae + (Kalophryninae + Cophylinae)) + (Asterophryninae + (Dyscophinae + Microhylinae)). 2 Genera not sampled: Pherohapsis was placed in a basal polytomy, Mantophryne was placed following Frost (2009). 3 Madecassophryne was not sampled in either of these, so was placed in a polytomy at the root. 4 Appendix S2: Representation of the global amphibian genus-level phylogeny. Figure S1 Representation of the genus-level global amphibian phylogeny, constructed as described in Appendix S1. Chosen clades (mostly families) are labelled above the branch leading to their most recent common ancestor. 5 6 7 8 9 Appendix S3: Results of all analyses rerun with the global amphibian phylogeny from Pyron & Wiens (2011). Spatial patterns for species richness when using the subset of amphibian species in the Pyron & Wiens tree were very similar to global species richness (Fig. S2a, Pearson correlation coefficient r = 0.984). Equally, spatial patterns for most of the phylogenetic diversity indices were similar when calculated on our tree (Fig. 1b–e) or on the Pyron & Wiens one (Fig. S2b–e) (see also Table 1 and main text). Values of Faith’s (1992) phylogenetic diversity (PD), total taxonomic distinctness (TTD) and average taxonomic distinctness (AvTD) across grid cells correlated well when calculated on the two different trees, but less so those for mean root distance (MRD) (Table 1, Fig. S3). Relationships of the different phylogenetic diversity indices with global species richness across grid cells did not change substantially when the Pyron & Wiens tree was used (Figs 1f–i, S2f–i). As with the results for our tree, we mapped residuals from the relationships of global species richness with Faith’s PD and TTD calculated on the Pyron & Wiens tree (Fig. S4; LOESS regressions with 11920.9 equivalent d.f.: Faith’s PD, residual sum of squares = 958.0; TTD, residual sum of squares = 21785.6). Spatial patterns of these residuals were closely correlated for the two phylogenies (Faith’s PD, r = 0.828; TTD, r = 0.851), and overlap of cells identified as unusual areas was high between the two phylogenies (Faith’s PD: 827 of 1179 cells, 70%; TTD: 763 of 1179 cells, 65%). 10 Figure S2 Global maps of (a) amphibian species richness for the subset of 2792 species in the Pyron & Wiens tree and (b–e) four indices of amphibian phylogenetic diversity calculated on the Pyron & Wiens phylogeny, and graphs (f–i) of the relationship of each phylogenetic diversity index with global species richness (6111 species in total) across grid cells. Phylogenetic diversity indices were: (b,f) Faith’s (1992) phylogenetic diversity (Faith’s PD), (c,g) total taxonomic diversity (TTD), (d,h) average taxonomic diversity (AvTD), and (e,i) mean root distance (MRD). Red lines in (f–i) were fitted to the data by local regression models with nonparametric smoothing. Colour scales in (a–e) are based on 30 equal-interval categories labelled with median values; the first and last categories are larger and labelled with the minimum and maximum value. Maps use the Behrmann projection. 11 12 Figure S3 Scatterplots of four indices of phylogenetic diversity calculated within grid cells on two different phylogenies, our global amphibian supertree and the Pyron & Wiens phylogeny: (a) Faith’s (1992) phylogenetic diversity (Faith’s PD), (b) total taxonomic diversity (TTD), (c) average taxonomic diversity (AvTD), and (d) mean root distance (MRD). Broken lines are the 1:1 line (intercept a = 0, slope b = 1). Solid lines are fitted regression lines as follows: (a) a = 12.45, b = 0.63, R2 = 0.95; (b) a = 9.52, b = 0.86, R2 = 0.94; (c) a = –3.98, b = 0.86, R2 = 0.85; (d) a = 0.03, b = 0.67, R2 = 0.56. 13 Figure S4 Global maps of residuals from a local regression model of (a) Faith’s (1992) phylogenetic diversity (Faith’s PD) and (b) total taxonomic diversity (TTD) against species richness, using the Pyron & Wiens phylogeny. Colour scales are based on equalinterval categories centred on zero and labelled with median values; the first and last categories are larger and labelled with the minimum and maximum value. Maps use the Behrmann projection. (a) (b) 14 REFERENCES Bossuyt, F., Brown, R.M., Hillis, D.M., Cannatella, D.C. & Milinkovitch, M.C. (2006) Phylogeny and biogeography of a cosmopolitan frog radiation: Late Cretaceous diversification resulted in continent-scale endemism in the family Ranidae. Systematic Biology, 55, 579-594. Cannatella, D.C. & Trueb, L. (1988) Evolution of pipoid frogs: morphology and phylogenetic relationships of Pseudhymenochirus. Journal of Herpetology, 22, 439-456. Che, J., Pang, J., Zhao, H., Wu, G., Zhao, E. & Zhang, Y. (2007) Phylogeny of Ranidae (Anura: Ranidae) inferred from mitochondrial and nuclear sequences. Molecular Phylogenetics and Evolution, 43, 1-13. Delorme, M., Dubois, A., Grosjean, S. & Ohler, A. (2006) The new ergotaxonomy of the family Megophryidae (Amphibia, Anura). Alytes, 24, 6-21. Faith, D.P. (1992) Conservation evaluation and phylogenetic diversity. Biological Conservation, 61, 1-10. Faivovich, J., Haddad, C.F.B., Garcia, P.C.A., Frost, D.R., Campbell, J.A. & Wheeler, W.C. (2005) Systematic review of the frog family Hylidae, with special reference to Hylinae: phylogenetic analysis and taxonomic revision. Bulletin of the American Museum of Natural History, 294, 1-240. Frost, D.R. (2009) Amphibian species of the world: an online reference. Version 5.3. American Museum of Natural History, New York. Available at: http://research.amnh.org/herpetology/amphibia/index.php (accessed May–June 2009). Frost, D.R., Grant, T., Faivovich, J., Bain, R.H., Haas, A., Haddad, C.F.B., De Sá, R.O., Channing, A., Wilkinson, M., Donnellan, S.C., Raxworthy, C.J., Campbell, J.A., Blotto, B.L., Moler, P., Drewes, R.C., Nussbaum, R.A., Lynch, J.D., Green, D.M. & Wheeler, W.C. (2006) The amphibian tree of life. Bulletin of the American Museum of Natural History, 297, 1-370. Fu, J. & Bi, K. (2007) A phylogeny of the high-elevation Tibetan megophryid frogs and evidence for the multiple origins of reversed sexual size dimorphism. Journal of Zoology, 273, 315-325. 15 Glaw, F. & Vences, M. (2006) Phylogeny and genus-level classification of mantellid frogs (Amphibia, Anura). Organisms Diversity & Evolution, 6, 236-253. Grant, T., Frost, D.R., Caldwell, J.P., Gagliardo, R., Haddad, C.F.B., Kok, P.J.R., Means, D.B., Noonan, B.P., Schargel, W.E. & Wheeler, W.C. (2006) Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae). Bulletin of the American Museum of Natural History, 299, 1-262. Guayasamin, J.M., Castroviejo-Fisher, S., Ayarzagüena, J., Trueb, L. & Vilà, C. (2008) Phylogenetic relationships of glassfrogs (Centrolenidae) based on mitochondrial and nuclear genes. Molecular Phylogenetics and Evolution, 48, 574-595. Hedges, S.B., Duellman, W.E. & Heinicke, M.P. (2008) New World direct-developing frogs (Anura: Terrarana): molecular phylogeny, classification, biogeography, and conservation. Zootaxa, 1737, 1-182. Köhler, F. & Günther, R. (2008) The radiation of microhylid frogs (Amphibia: Anura) on New Guinea: a mitochondrial phylogeny reveals parallel evolution of morphological and life history traits and disproves the current morphology-based classification. Molecular Phylogenetics and Evolution, 47, 353-365. Li, J., Che, J., Bain, R.H., Zhao, E. & Zhang, Y. (2008) Molecular phylogeny of Rhacophoridae (Anura): a framework of taxonomic reassignment of species within the genera Aquixalus, Chiromantis, Rhacophorus, and Philautus. Molecular Phylogenetics and Evolution, 48, 302-312. van der Meijden, A., Vences, M., Hoegg, S., Boistel, R., Channing, A. & Meyer, A. (2007) Nuclear gene phylogeny of narrow-mouthed toads (Family: Microhylidae) and a discussion of competing hypotheses concerning their biogeographical origins. Molecular Phylogenetics and Evolution, 44, 1017-1030. Ohler, A. & Dubois, A. (2006) Phylogenetic relationships and generic taxonomy of the tribe Paini (Amphibia, Anura, Ranidae, Dicroglossinae), with diagnoses of two new genera. Zoosystema, 28, 769-784. Olalla-Tárraga, M.Á., McInnes, L., Bini, L.M., Diniz-Filho, J.A.F., Fritz, S.A., Hawkins, B.A., Hortal, J., Orme, C.D.L., Rahbek, C., Rodríguez, M.Á. & Purvis, A. (2011) Climatic niche conservatism and the evolutionary dynamics in species range 16 boundaries: global congruence across mammals and amphibians. Journal of Biogeography, 38, 2237-2247. Pyron, R.A. & Wiens, J.J. (2011) A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Molecular Phylogenetics and Evolution, 61, 543-583. Scott, E. (2005) A phylogeny of ranid frogs (Anura: Ranoidea: Ranidae), based on a simultaneous analysis of morphological and molecular data. Cladistics, 21, 507574. Smith, S.A., Arif, S., Nieto Montes de Oca, A. & Wiens, J.J. (2007) A phylogenetic hot spot for evolutionary novelty in Middle American treefrogs. Evolution, 61, 20752085. Stuart, B.L. (2008) The phylogenetic problem of Huia (Amphibia: Ranidae). Molecular Phylogenetics and Evolution, 46, 49-60. Van Bocxlaer, I., Roelants, K., Biju, S.D., Nagaraju, J. & Bossuyt, F. (2006) Late Cretaceous vicariance in Gondwanan amphibians. PLoS ONE, 1, e74. Van Bocxlaer, I., Loader, S.P., Roelants, K., Biju, S.D., Menegon, M. & Bossuyt, F. (2010) Gradual adaptation toward a range-expansion phenotype initiated the global radiation of toads. Science, 327, 679. Vieites, D.R., Min, M. & Wake, D.B. (2007) Rapid diversification and dispersal during periods of global warming by plethodontid salamanders. Proceedings of the National Academy of Sciences USA, 104, 19903-19907. Wiens, J.J., Kuczynski, C.A., Duellman, W.E. & Reeder, T.W. (2007a) Loss and reevolution of complex life cycles in marsupial frogs: does ancestral trait reconstruction mislead? Evolution, 61, 1886-1899. Wiens, J.J., Parra-Olea, G., García-París, M. & Wake, D.B. (2007b) Phylogenetic history underlies elevational biodiversity patterns in tropical salamanders. Proceedings of the Royal Society B: Biological Sciences, 274, 919-928. Wiens, J.J., Sukumaran, J., Pyron, R.A. & Brown, R.M. (2009) Evolutionary and biogeographic origins of high tropical diversity in Old World frogs (Ranidae). Evolution, 63, 1217-1231. 17 Wiens, J.J., Kuczynski, C.A., Hua, X. & Moen, D.S. (2010) An expanded phylogeny of treefrogs (Hylidae) based on nuclear and mitochondrial sequence data. Molecular Phylogenetics and Evolution, 55, 871-882. Wilkinson, M. & Nussbaum, R.A. (2006) Caecilian phylogeny and classification. Reproductive biology and phylogeny of Gymnophiona (caecilians) (ed. by J.-M. Exbrayat), pp. 39-78. Science Publishers, Enfield, NH. Wollenberg, K.C., Vieites, D.R., van der Meijden, A., Glaw, F., Cannatella, D.C. & Vences, M. (2008) Patterns of endemism and species richness in Malagasy cophyline frogs support a key role of mountainous areas for speciation. Evolution, 62, 1890-1907. Zhang, P. & Wake, M.H. (2009) A mitogenomic perspective on the phylogeny and biogeography of living caecilians (Amphibia: Gymnophiona). Molecular Phylogenetics and Evolution, 53, 479-491. Zhang, P., Papenfuss, T.J., Wake, M.H., Qu, L. & Wake, D.B. (2008) Phylogeny and biogeography of the family Salamandridae (Amphibia: Caudata) inferred from complete mitochondrial genomes. Molecular Phylogenetics and Evolution, 49, 586-597. Zhang, P., Chen, Y., Zhou, H., Liu, Y., Wang, X., Papenfuss, T.J., Wake, D.B. & Qu, L. (2006) Phylogeny, evolution, and biogeography of Asiatic salamanders (Hynobiidae). Proceedings of the National Academy of Sciences USA, 103, 73607365. 18