POLA_24864_sm_suppinfo
advertisement

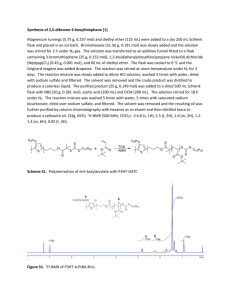
Supporting Information Synthesis of Multi-armed Poly(3-hexyl thiophene) Star Polymer with Microgel Core by GRIM and ATRP Methods Hyun-Ji Kim,1,2 Yun-Jae Lee,1 Seung Sang Hwang,1 Dong Hoon Choi,2 Hoichang Yang,3 and KyungYoul Baek1* 1 Polymer Hybrids Center, Korea Institute of Science and Technology, Seoul 136-791, Korea 2 3 Department of Chemistry, Korea University, Seoul 136-701, Korea Department of Advanced Fiber Engineering, Inha University, Incheon 402-751, Korea General synthetic procedure for poly(3-hexylthiophene) (P3HT) macroinitiator (Scheme 1). Scheme S1. Synthesis of CPA end group functionalized poly(3-hexylthiophene) (P3HT) macroinitiator (3) for Ru catalyzed living radical polymerization. Materials. All reactions were conducted under purified argon pressure. 2,5-dibromo-3hexylthiophene was synthesized according to the published literature[1]. The other chemicals were purchased from Aldrich Chemical Co., were used without further purification unless mentioned otherwise: [1,3-bis(diphenylphosphino)propane]dichloronickel(II) (Ni(dppp)Cl2, 98 %); tert1 butylmagnesium chloride (2.0 M in diethylether); allylmagnesium bromide (1.0 M in diethylether); 9-borabicyclo[3.3.1]nonane (9-BBN, 98 %); triethylamine (TEA, 99.5 %); α-chlorophenylacetyl chloride (CPA, 90 %). Hydrogen peroxide (H2O2, > 30 %) was purchased from Daejung, South Korea. The solvents, methylene chloride, dimethylformamide (DMF), tetrahydrofuran (THF) and diethylether were purchased as HPLC grades, were dried overnight over calcium chloride and purified by distillation CaH2 under reduced pressure. All reactions were performed inside the glove box (moisture < 1 ppm and O2 < 1 ppm). (a) Synthesis of allyl terminated poly(3-hexylthiophene) (1):[2] The mole ratio of various components were [monomer]: [t-BuMgCl]: [Ni(dppp)Cl2]: [AllylMgBr] = 1 : 1 : 0.011 : 0.3. To an oven dried one-neck 250 mL r.b. flask equipped with a stirring bar was introduced monomer (4.0g, 12.27 mmol) and 24 mL of dry THF. Required amount of tert-butyl magnesium chloride (6.13 mL in 2.0 M in diethylether, 12.27 mmol) was added to the flask via degassed syringe and mixture was stirred for 3 h. Thereafter, solution mixture was diluted by 120 mL of dry THF followed by addition of Ni(dppp)Cl2 (0.0731 g, 0.135 mmol). Immediately the color of reaction mixture turned bright orange and changed to dark orange after the addition of 1.0 M of allyl magnesium bromide (3.68 mL, in 1.0 M in diethylether 3.68 mmol) via degassed syringe. The mixture was stirred for an additional 2 min; removed reaction flask and precipitated into ten-fold excess of methanol. The polymer (purple color) was filtered, using Millipore (1.0 μm) PTFE filter paper, and purified using Soxhlet extraction using n-pentane for 12 h. Finally, polymer (yield = 1.2 g) was dried under vacuum at 40 °C for 24 h and analyzed by 1H NMR, GPC (Mn = 15,300; Mw/Mn = 1.13) and MALDI-TOF-MASS (Mn = 9,000 Da; ; Mw/Mn = 1.05) (see Figure S1A, Figure S2A and Figure S3A for more details). (b) Synthesis of hydroxypropyl terminated poly(3-hexylthiophene) (2):[3] To a one-neck oven dried r.b. flask equipped with stirring bar was transferred allyl terminated P3HT (1) (1.1 g, Mn = 15,200, 0.07mmol) followed by addition of dry THF (120 mL) in 250mL of two-neck 250 mL r.b. flask. After the addition of 9-BBN (0.176 g, 0.74 mmol), the reaction flask was taken outside and stirred for 48 h in an oil bath maintained at 40 °C. Thereafter, 6 M solution of NaOH (0.33 mL) was added to the reaction flask, followed by removal of oil bath and stirring continued for an additional 15 min. Upon cooling, added 33 % aqueous solution of hydrogen peroxide (0.35mL), and reaction stirred for 12 h at 40 °C. The polymer was precipitated in ten-fold excess of methanol-water (1:1) 2 mixture. The polymer was filtered using Millipore (1.0 μm) PTFE filter paper and purified using Soxhlet extraction using methanol for 12 h. Finally, polymer (Yield = 1.1 g) was dried under vacuum at 40 °C for 24 h and analyzed by 1H NMR, GPC (Mn = 16,100; Mw/Mn = 1.11) and MALDI-TOF-MASS (Mn = 9,100 Da; Mw/Mn = 1.05) (see Figure S1B, Figure S2B and Figure S3B for more details). (c) Synthesis of α-chlorophenylacetyl (CPA) terminated poly(3-hexylthiophene) (3):[4] To two-neck oven dried r.b. flask equipped with stirring bar was transferred hydroxypropyl terminated P3HT (2) (1.0 g, MnSEC = 15,600, 0.06mmol) followed by addition of 150 mL of anhydrous THF. The reaction flask, equipped with a three-way stop cock and condenser, was taken outside and stirred for 15 min in an oil bath maintained at 40 °C. To this was added TEA (1.31 mL, 9.42 mmol) and drop-wise addition of CPA (1.36 mL, 8.59 mmol) for five minutes. Thereafter the reaction mixture was stirred for 12 h at 40 °C. The P3HT macroinitiator was precipitated in ten-fold excess of methanol. The polymer was filtered using Millipore (1.0 μm) PTFE filter paper, followed by washing with 100 mL of cold methanol and purified using Soxhlet extraction using methanol for 12 h. Finally, polymer (Yield = 0.8 g) was dried under vacuum at 40 °C for 24 h and analyzed by NMR, GPC (Mn = 15,300; Mw/Mn = 1.13) and MALDI-TOF-MASS (Mn = 9,200 Da; ; Mw/Mn = 1.06) (see Figure S1C, Figure S2C and Figure S3C for more details). 3 Figure S1. GPC curves of end-functionalized poly(3-hexylthiophene)s: (A) allyl terminated P3HT (1); (B) hydroxypropyl terminated P3HT (2); (C) CPA terminated poly(3-hexylthiophene) (3). Figure S2. MALDI-TOF-MASS spectra of end-functionalized poly(3-hexylthiophene)s: (A) allyl terminated P3HT (1); (B) hydroxypropyl terminated P3HT (2); (C) CPA terminated poly(3hexylthiophene) (3). 4 (A) A h S Br a b d, e, f j g n i c d e f b c g a h j i B (B) h S Br a b g d, e, f l OH n k c d e b,h f c g k l C (C) h S Br a b n k O m o p n O g d, e, f q Cl c d e b,h f c g p,q o n 8 7 6 m 5 4 k 3 2 1 ppm Figure S3. 1H NMR spectra of end-functionalized poly(3-hexylthiophene)s in CDCl3: (A) allyl terminated poly(3-hexylthiophene) (1); hydroxypropyl terminated poly(3-hexylthiophene) (2) (B); CPA terminated poly(3-hexylthiophene) (3) (C). 5 4000 3000 2000 1000 Figure S4. FT-IR spectra of the EGDMA crosslinker (A), the rr-P3HT-CPA macroinitiator (B), and the rr-P3HT star polymer (C) obtained after addition of EGDMA in 15 h. See Figure 2. REFERENCES [1] Chaloner, P. A.; Gunatunga, S. R.; Hitchcock, P. B. J. Chem. Soc., Perkin Trans. 1997, 2, 1601. [2] Jeffries-EL, M.; Sauve, G.; McCullough, R. D. Adv. Mater. 2004, 16, 1018. [3] Iovu, M. C.; Zhang, R.; Cooper, J. R.; Smilgies, D. M.; Javier, A. E.; Sheina, E. E.; Kowalewski, T.; McCullough, R. D. Macromol. Rapid Commun. 2007, 28, 1819. [4] (a) Baek, K.-Y.; Kamigaito, M.; Sawamoto, M. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 1937. (b) Baek, K.-Y.; Kamigaito, M.; Sawamoto, M. J. Polym. Sci. Part A: Polym. Chem. 2002, 40, 1972. 6