The Balloon Inflator
advertisement

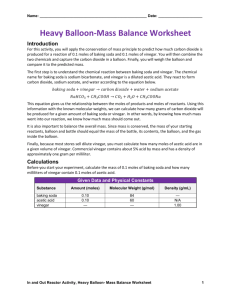
The Balloon Inflator Grade 5 Strand: Understanding Matter and Energy Topic: Properties of Changes in Matter Expectations: 2.3 Using scientific inquiry/ experimentation skills to investigate changes of state and changes in matter 3.5 Describe chemical changes in matter as changes that are irreversible Required Materials: 60mL of vinegar 1 balloon 1 empty bottle 1 funnel 30mL of water 5mL of baking soda Procedure: Pour the water into the bottle Pour the vinegar into the bottle Place the cap onto the bottle and shake well (to mix the two substances together) Stretch out the opening of the balloon and use the funnel to put the baking soda into the balloon Remove the cap from the bottle Without letting the baking soda leak out into the liquid in the bottle, seal the bottle with the balloon Lift the balloon to allow the baking soda to fall into the liquid Scientific Explanation (for a student in grade 5): The baking soda is a base and the vinegar is an acid. An acid is a substance that tastes sour and it dissolves in water. A base is a substance that feels slippery. By mixing these two ingredients together, we create an acid-base reaction (neutralization). This is a type of chemical reaction and it is not reversible. We cannot extract our original ingredients through the gas (carbon dioxide) we created. The balloon is a visual representation to help show the gas created by the chemical reaction. The result of this reaction created water, salt, and a gas. The water and the salt are created from the neutralization reaction of mixing the acid and the base together. The carbon dioxide (the gas) is created because the vinegar and the baking soda are not pure acid and base. Opportunities and Considerations: To enhance the students’ learning, the students can measure the time it takes for the balloon to inflate at different water temperature. The students can also see if the size of the bottle affects the time it takes for the balloon to inflate. If students are handling the substances, they must wear gloves and eye protection. Since there is a chemical reaction, students must be aware of the possible risks involved with this activity. For instance, the balloon might fly off and injure them; therefore, students must be responsible when working with these substances. For students with a latex allergy, the balloon can be replaced with a sandwich bag sealed at the bottle with an elastic band. References: Loeschnig, L. (2000). Giant Book of Science Fun. New York: Sterling Publishing Company. Presenters: Candy Mak and Katie Weiler-Dean