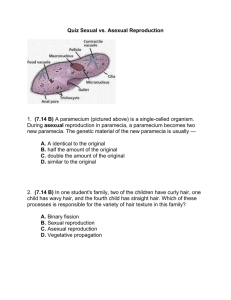
Paramecia
advertisement

Cigarette Toxin Effect on Paramecia tetraurelia Cultures By: Skyler D. Resendez Lab Partner: Thomas Heath Bio 305 – Genetics 11/19/11 Introduction Paramecium tetraurelia: In this study, microorganisms known as Paramecia tetraurelia were experimented upon. Paramecia are protozoa covered in alternating cilia and trichocysts. As with any organism in the Paramecium genus, they are unicellular eukaryotes, approximately 100 micrometers in length, yet they are complex enough to be capable of showing an array of information during biomedical research (P ar a m e c i u m te t r a u r e l i a , 2 0 1 1 ) . Movement: The movement of the Paramecia is very fluid. The organisms turn around quickly in a motion that looks as if they’re folding over. Due to their movement pattern, Paramecia have often been called “swimming neurons.” These microorganisms are able to sense changes in the environment. Paramecia swim forward when they are in a state of homeostasis. However, when that state of homeostasis is compromised, the response is a flooding of calcium ions into the organism. The calcium channels are located at the base of the cilia. This causes the Paramecium to go backwards. This is known as an avoidance reaction (E u k a r y o t es g e n o me s - p a r a m e c i u m t e tr a u r el i a , ( n . d . ) , P a r a me c i u m te t r a ur e li a , 2 0 1 1 ,) . “Junk” DNA: Paramecium tetraurelia are “prime” organisms for research purposes. This is due to a number of facts, including their small amount of what is often referred to as “junk” DNA (especially when compared to primates.) Junk DNA simply refers to segments of DNA that do not code for anything. Primates, such as humans, have a large amount of Junk DNA. This is partially due to what are known as “jumping genes,” or transposons. Transposons, which are actually segments of DNA (not genes), have the ability to move from place to place on the chromosomes. Transposons lengthen the amount of “junk” DNA in primates, which partially accounts for the massive percentage of non-coding DNA in humans. Approximately 96 percent of a human’s DNA falls under this category. This is why it is much simpler to cause a mutation in Paramecium tetraurelia. The coding DNA of the Paramecia accounts for about 70% of their genome. In order for a mutation to be seen, the change in the genome must occur in a section of coding DNA. Therefore, any mutations that occur in the Paramecia have a 70% chance of showing, compared to the measly 4% chance in humans. Note that 4% is a high estimate. Food Supply: As it is well known that Paramecium tetraurelia are auxotrophs, they need a steady food supply and supportive environment for them to continue to prosper. Paramecia often prey upon different bacteria. Thus, they were constantly supplied with a sufficient amount of Klebsiella pneumoniea. The organism Klebsiella pneumonia is a gram-negative bacterium and is also the pathogen often responsible for urinary-tract infections. Klebsiella are rod-shaped and are facultative anaerobic bacteria, the latter of which meaning that they will utilize oxygen when available, but can survive without it (Klebsiella pneumoniae, (n.d.)). The Klebsiella pneumonia cultures were put into a wheat grass solution in order for the bacteria to have a sufficient nutrient supply. With the Klebsiella feeding off the wheat grass and the Paramecia feeding off the Klebsiella, a food chain had been produced. The food supplies were constantly reintroduced to keep the Paramecia in top condition. Methods and Materials Maintaining Paramecia Cultures In this experiment, it was necessary to have a constant source of healthy Paramecia. In order to do this, the researchers were constantly aiding and altering the environment of the microorganisms. This is necessary to keep nutritional sources for the Paramecia at adequate levels and to keep waste products from filling up their limited environment. In order to first acquire two stock solutions of Paramecia, the researchers acquired two flasks that contained approximately 10 mL of premade wheat media solution in each (the size of the flasks isn’t of too much concern as long as there is enough room within the flask to hold the wheat media and small amounts of Paramecia that will be added). It was then necessary for the conductors of the experiment to inoculate the wheat media with Klebsiella pneumoniae. An agar plate that had multiple colonies of the Klebsiella was then pulled from the refrigerator. The parafilm keeping the lid fastened tightly to the agar plate was then removed as the researchers acquired an inoculating loop. The inoculating loop was then heated to high temperatures via a bacti-cinerator for 10 seconds and then allowed to cool for 20. This process helps to sterilize the equipment to prevent contamination. The inoculating loop was then used to lightly swipe a colony from the agar plate and then was put directly into the wheat media and stirred. This process was done for both of the flasks and then the flasks were put into an incubator over night. An alternative method to this would have been to simply leave the flasks on the shelf for two days, rather than one day in the incubator. The following day, the flasks were removed from the incubator and given five to ten minutes to cool off. Once the flasks and their contents reached approximate room temperature, the researchers acquired stock flasks of previously created Paramecia cultures. Micropipettes were used to transfer 500 microliters (uL) of the stock Paramecia solution into each of the flasks containing Klebsiella and wheat grass solution. Using this process, the Paramecia cultures reached their prime approximately six to nine days afterwards and began dying at around day fourteen. With this in mind, the researchers repeated this entire process weekly, requiring two more flasks of wheat media. From this point on, the older flasks containing Paramecia were checked using a microscope after micropipetting 100 uL of their contents from each onto a microscope slide. If they contained healthy, viable cells, 500 uL were taken from each and put into the two newer flasks. A week later, the newer flasks were checked in a similar manner, and if they too contained healthy cells, the older flasks (now two weeks old) were discarded and the newer flasks were used as the stock samples to repeat this whole process again. Note: All flasks were labeled thoroughly throughout the course the experiment to ensure that they could be found and that their contents were known. Creating a Stock Cigarette Solution The entire goal of this experiment was to cause visible mutations in the Paramecia cultures. These mutations could have included changes in size (length/width), shape, movement pattern, general structure (ex. black dots at the ends of the cells), etc. To do this, the researchers decided to use cigarette toxins. With this concept in mind, it was necessary to find a way to expose the Paramecia to such toxins. To do this, the researchers made a sort of “cigarette tea” and considered it their 100% stock solution. The researchers acquired wheat media and cigarettes (the brand “West” was used) to craft such a solution. Fifty mL of wheat media was measured out using a pipette. The media was put into a glass container with a lid. The individuals crafting the experiment then tore apart five cigarettes and put the contents into the container, excluding the filter and papers. This achieved the desired goal of having a stock solution that is one cigarette per 10 mL of wheat media. This mixture was then allowed to sit over night. The following day, the container was brought back into the lab. At this point in time, the cigarette stock solution was poured out into a beaker (150 mL). A petri dish lid was then put over the top of the beaker to try to keep too much of the liquid portion of the solution from evaporating out, as the next step was to heat it to a boil. To do this, a hot plate was used. It did not take long for the media to start boiling. This was done in hopes of further extracting the toxins into the liquid portion of the solution. The researchers then acquired a small side-arm flask, filter paper, a Buchner funnel of appropriate size, and parafilm. The filter paper was put inside of the Buchner funnel, and the draining portion of the Buchner funnel was put into the side arm flask. The parafilm was used to make an air tight seal around the funnel and side-arm flask, but an appropriately sized stopper would have probably been more effective. The side arm flask was then attached via a hose to a vacuum apparatus used in vacuum filtration. The vacuum was turned on and the cigarette solution was then slowly decanted into the funnel. The pores in the funnel would get clogged easily, so it was necessary to remove the funnel of its contents and change the filter paper often. Disposable pipettes were very helpful with this process. Disposable rubber gloves were also used to squeeze some of the liquid out of the tobacco which was very good at absorbing (and not releasing) the media. Through decanting, repeated filter changes, and slow vacuum filtration, a decent yield of cigarette stock solution was obtained. After this process was complete, it was noted that the solution contained small, red rubber chunks. The chunks must have been from the inside of the deteriorating hose. This was a simple fix requiring a funnel, Kimwipes, and a conical tube. The Kimwipe was put into the funnel and the funnel into the tube. The solution (now free of all chunks besides the rubber pieces) easily filtered through the Kimwipes, leaving the rubber chunks behind. The cigarette stock solution was then poured carefully into a beaker, covered with parafilm, labeled, and put onto a shelf. Creating Paramecia/Stock Cigarette Solution Mixes When it came time to begin attempting to cause mutations, the cigarette stock solutions, the Paramecia stock solutions (at this point being seven days old and in their prime), and a well plate were acquired. The well plate already had its rows and columns labeled with letters and numbers. The amount of solution in which each well could hold was determined using water and pipettes and a volume of 500 uL was decided upon per sample. The concentrations created were 15% cigarette stock solution and 85% Paramecia solution, 20% cigarette stock solution and 80% Paramecia solution, and lastly, 25% cigarette stock solution and 75% Paramecia solution (from this point on the concentrations will simply be referred to by their cigarette solution concentration.) The formula C1V1 = C2V2 was used to figure out the volumes needed. Ex. C1 = original cigarette solution concentration (100%); V = volume of cigarette solution needed; C2 = desired concentration (for this example, 15%); V2 = final volume (500 uL) C1V1 = C2V2 100% (V1) = (15%) (500uL) 100% (V1) = 7500 % uL (V1) = 75 uL Therefore, 75 uL of stock cigarette solution was mixed with 425 uL of Paramecia solution to get a 15% concentration. With this in mind, the 15% concentrations were composed of 75 uL of stock cigarette solution mixed with 425 uL of Paramecia solution, the 20 % concentrations were composed of 100 uL of stock cigarette solution mixed with 400 uL of Paramecia solution, and the 25% concentrations were composed of 125 uL of stock cigarette solution mixed with 375 uL of Paramecia solution. Stock wells were also created (500 uL of stock Paramecia solution) for comparison. Wells A1-A3 were designated to the stock solutions, B1-B3 were designated to the 15% concentrations, C1-C3 were designated to the 20% concentrations, and D1-D3 were designated to the 25% concentrations. All of these concentrations were created with the use of micropipettes. The wells and flasks were then put back on the shelf. The wells were then to be left alone for three days. Observing Results and Reintroducing a Suitable Environment After three days, the Paramecia were checked once more with a microscope using the techniques described previously. They were compared to the stock solutions in the wells. Abnormalities were noted and any mutated cells (possible mutation examples are described above) were then transported to a suitable environment using pipettes (it was made sure that only Paramecia that had been residing in the same concentration of cigarette solution were put together in flasks). Such an environment consisted of Klebsiella/wheat grass solutions crafted in the same way described before. The making of such environments needed to be done ahead of time because the incubation step requires at least a day and four days in cigarette concentrations of 15, 20, and 25% is sure to kill the Paramecia. If the Klebsiella/wheat grass solutions had to be crafted more than a day prior, they could simply be refrigerated after incubation to keep the Klebsiella from becoming too numerous. Results Throughout the course of this experiment, the researchers managed to force multiple changes on the Paramecia. The scientists documented altered features in the 20% cigarette, 80% Paramecia cultures over time as they were continually put into new environments for them to thrive. The changes shifted in frequency, severity, and type over time. Each time the cultures were checked under a microscope, any abnormal features were documented and analyzed. Changes such as abnormalities in size, shape, motility, and other features were documented on the following dates: 17 October, 2011 On this particular date, the Paramecia cultures that were observed had been sitting in the 20% cigarette solution for a total of three days. In this batch, the alterations to the Paramecia were plentiful. There were cells with the black dots, usually associated with the ends of the cells, dispersed throughout the entire organism. (The percentage was calculated by taking the area of the organism and dividing it by the area that the black dots covered within the organism. See Table 3 and Figure 6 for data regarding black dot coverage of the Paramecia.) Abnormal movement was also documented. Rather than the usual “folding-over” motion of the Paramecia, they seemed to be rotating about a point. Based off of observations taken from a 2 minute long video with multiple cells rotating in such a manner (N = 7, where N is the number of cells observed), the researchers estimated that the Paramecia were rotating at a rate of approximately three spins every two seconds. Some cells were also quite misshapen and many were smaller than usual. (See Table 1 and Figures 3 and 4 for data regarding the size of the Paramecia.) 20 October, 2011 The Paramecia observed on this date were cells that had been transferred from the wells and put into a flask of wheat grass/Klebsiella solution. By this point, the cells had lived in their new environment for a total of three days. The researchers made sure to note the fact that there was still cigarette concentration within this flask, but it was narrowed down to a very slight amount as there was very little solution from the wells being put into a large volume of wheat grass/Klebsiella solution. When checked, the sample was full of life, much of which was distorted anatomically once again. The most noticeable change in the cells was that many of them had one very narrow end that looked “cone-like.” (See Table 4 for data regarding the “cone-like” Paramecia.) Another strange phenomenon that was noted was that some of the Paramecia exhibited what seemed to be an inability to break apart right away at the end of asexual reproduction. This was a frequent occurrence as two cells would be stuck together at a single point, many of which were moving about, the cell in the back simply being dragged along. (See figure 1 for a photograph taken of two Paramecia stuck together in such a fashion.) It seemed as if there were always one or two pairs in frame while looking at approximately 40 cells at a time. The researchers also noted that the cells could primarily be found around what seemed like chunks of cigarette toxin/content. (See Table 2 and Figure 5 for data regarding approximate percentages of Paramecia that were located in the chunks of the wheat grass media.) Figure 1: This is a 100x magnified (rescaled) photo taken on October 20th, 2011. The photo is of multiple Paramecia and a few of their respective lengths (in um) and areas (in um^2). The lengths of these Paramecia are particularly interesting due to how small they truly are compared to average Paramecia (the average length for the control group on this day was 108.697 um). Another interesting portion of this photo is the two cells that are stuck together toward the bottom of the picture. This is the change noted where they had difficulty finishing asexual reproduction. The researchers coined the term “Gemini” as a possible name for such Paramecia. 24 October, 2011 The Paramecia observed on this date were from the same environment that they were in on the 20th of October. Despite this, their abnormalities still changed between the two dates. These observations were made just prior to putting 500 um of the culture into a new environment for further study to be done a few days later. The Paramecia in this flask were altered, but again, the changes were slightly different from the previous date’s observations. It was noted that the cells still exhibited the “cone-shaped” ends that had been seen before, but this was to a much lesser extent. The Paramecia were also smaller in size and area than the control group, but the cells began to look relatively more average anatomically than they appeared to be on the 20th of October. 27 October, 2011 This was the final date the researchers had set aside for observations. The Paramecia observed on this date had been in their environment for a total of approximately three and a half days. The researchers had transferred cells from the old flask into another new Klebsiella/wheat grass solution earlier that week, once again lowering the cigarette toxin concentration even further (at this point, the cigarette toxin levels were almost negligible). The changes in the Paramecia were much less frequent on this date than they had been during previous observations. However, there were still cells that were abnormal anatomically. Misshapen Paramecia were observed including one that had a “bubbling membrane.” (See Figure 2 for a photograph taken of a misshapen Paramecium on this date.) The researchers also managed to catch cells in the act of conjugation. The conjugation between the cells was to help increase genetic diversity. The scientists observed this occurrence multiple times within the culture, indicating that mutations had occurred and the cells were trying to rectify the situation. Lastly, abnormal movement was once again documented, this time a strange twisting sort of motion from cells that often died directly afterwards. Based on a minute long video, the cells seemed to twist over completely (360 degrees) once every 2.8 seconds (on average). The cells seemed to spiral until they met their demise. Figure 2: This is a 40x magnified (rescaled) photo taken on October 27th, 2011. The picture is of two Paramecia and their respective lengths (in um). This photo was chosen because of the comparison of the fairly average Paramecium on the right, versus the small, abnormally shaped, altered cell on the left. These were just a few of the changes seen throughout the course of this experiment. Table 1: This data table shows the average sizes of Paramecia of which the researchers took pictures, using an N number of 12 for both the control and experimental groups. Average Sizes of Paramecia by Date – Table 1 Length (um) Area (um^2) Control Experimental Control Experimental 17-Oct 112.421 93.151 3145.651 2331.854 20-Oct 108.697 97.563 3110.431 2541.187 24-Oct 118.563 99.911 3001.647 2275.108 27-Oct 111.652 103.86 3043.647 2226.562 The software program “ImageJ” was used with a standard micrometer as a scale to find both lengths (in um) and area (in um^2). The data shown in Table 1 is a result of numerous measurements taken and averaged from each day and shows that the experimental cells were often considerably smaller than their control groups. 140 120 100 80 60 40 20 0 Length in um Experimental 27-Oct 26-Oct 25-Oct 24-Oct 23-Oct 22-Oct 21-Oct 20-Oct 19-Oct 18-Oct Length in um Control 17-Oct Length (um) Length of Paramecia on Various Dates - Figure 3 Dates Figure 3: This graph gives a visual of some of the information displayed in Table 1. It is a good visual to see the difference between the average lengths of the control group versus the average lengths of the experimental group on various dates. An N number of 12 was used for both the control and experimental groups. 4000 3500 3000 2500 2000 1500 1000 500 0 27-Oct 26-Oct 25-Oct 24-Oct 23-Oct 22-Oct 21-Oct 20-Oct 19-Oct 18-Oct Area in um^2 Control 17-Oct Area (um^2) Area of Paramecia on Various Dates Figure 4 Area in um^2 Experimental Dates Figure 4: This graph gives a visual of some of the information displayed in Table 1. It is a good visual to see the difference between the average areas of the control group versus the average areas of the experimental group on various dates. The difference in average area between the two groups can be quite drastic. An N number of 12 was used for both the control and experimental groups. Table 2: This data table shows approximate percentages of Paramecia that were located in the chunks of the wheat grass media, using an N number of 31 for both the control and experimental groups. Approximate Percentages of Paramecia in Solid Portions of Wheat Media – Table 2 Approximate Percentages Date Control Experimental 20-Oct 13% 90% 24-Oct 15% 85% 27-Oct 12% 40% This solid portion of the wheat media seemed to have absorbed much of the cigarette toxin and was often darker in appearance than in the control cultures. Due to this fact, the altered Paramecia were often found in much higher frequencies in the solid portion than in the rest of the media. These percentages were approximated based on numerous pictures of Paramecia located both in and out of Approximate Percentage these sections of the media. Approximate Percentages of Paramecia in Solid Portions of Wheat Media - Figure 5 120% 100% 80% 60% 40% 20% 0% Control Experimental Dates Figure 5: This graph displays visually the information from Table 2. It shows that the Paramecia in the experimental cultures were found gathering in and around the solid portion of the wheat media at a much higher frequency than those of the control culture. It also shows that this trend decreased over time as the Paramecia repaired their DNA through practices such as conjugation. An N number of 31 was used for both the control and experimental groups. Table 3: Table 3 shows numerically the average percentage of how much each microorganism was covered with the black dots, using an N number of 14 for both the experimental and control groups. Black Dot Coverage Percentages – Table 3 Experimental Control 17-Oct 76% 23% 20-Oct 64% 26% 24-Oct 48% 27% 27-Oct 44% 24% Usually the black dots are located only at the ends of each organism and thus, they cover a much smaller amount of the organism’s entirety. These percentages were acquired by dividing the entire area of a Paramecium by the area taken up by the black dots within the microorganism. That number was multiplied by 100%. This was done for multiple Paramecia from numerous pictures on each date. Black Dot Coverage Percentages Figure 6 80% 60% 40% Experimental 20% Control Dates 27-Oct 26-Oct 25-Oct 24-Oct 23-Oct 22-Oct 21-Oct 20-Oct 19-Oct 18-Oct 0% 17-Oct Percentages 100% Figure 6: This graph shows visually the information from Table 3. It is plain to see that the black dots covered a greater percentage of the microorganisms in the experimental cultures than they did in the control cultures (on average). It is also possible to see the declining slope in percentages of the experimental group over time, once again indicating that DNA repair had occurred. An N number of 14 was used for both the control and experimental groups. Table 4: This data table attempts to quantify how extreme the “cone-shaped head” phenomenon was on average, using an N number of 13 for both the control and experimental groups. Average End Width Measurements – Table 4 Average Width of Head Average Width of Tail End (um) End (um) Differences (um) Control Experimental Control Experimental Control Experimental 20-Oct 9.838 6.545 11.547 12.499 1.709 5.954 24-Oct 9.906 7.488 11.803 11.384 1.897 3.896 This was done using comparisons. The researchers acquired the average width of each end of the Paramecia in each culture on the two dates that the phenomenon had occurred (the widths were taken approximately 5 um from the outermost point of the cell membrane.) The average differences between the corresponding head/tail measurements (the head end being the one that was not as wide) were then found. By looking at the average differences and noting the generally unwavering widths of the tail end, one could clearly draw the conclusion that the experimental Paramecia had narrower heads. It is also important to note that the phenomenon decreased in intensity between October 20th and October 24th. Discussions The data throughout this experiment and the statistic analysis of it consistently indicated that mutations occurred. There was the presence of anatomic abnormalities including changes in the width at one end, general size decreases, both in length and area, black dot dispersal, and general shape. There were also changes in movement and abnormal behavior such as tending to stay close to where the toxin was most concentrated. All of this was observed, analyzed, and quantified, and the numbers don’t lie. The large amount of cells attempting to conjugate also indicates that mutations occurred. This is because the Paramecia were attempting to increase biodiversity to help remove or rectify any negative attributes attained due to the changes caused by the cigarette toxin. This experiment has also shown how effective these organisms are at repairing their own DNA as the percentage and degree of changes observable within the experimental groups decreased over time. However, it is important to always remain somewhat skeptical. There are always alternative possibilities to what may have occurred. It was observed that the altered cells were often found around the visible chunks of what appeared to be the cigarette toxins. One might argue that the reason the number of altered Paramecia dropped and the amount of average cells steadily increased is simply because the abnormal cells were not passing on the genetic coding for such alterations. This could have occurred for a number of reasons including that they may have been dying before reproducing, whereas the ones who avoided the toxin and remained anatomically average did reproduce. The different mutations that were observed may have simply been due to non-mutated offspring coming into contact with the toxins since it was impossible to fully eliminate said chemicals from the solution. However, the concentration of cigarette toxin drastically decreased after every transfer to a new environment. If this were the case entirely, it would mean that second generation mutated offspring never came about. This is extremely doubtful, though it may have contributed to some of the observed changes in the Paramecia. It is much more believable that there was a large degree of mutated offspring being born and that DNA repairing occurred as these amazing organisms conjugated, but it is important to keep a critical, ever-questioning mindset. Seeing changes that were quite frequent wane almost out of existence is a good indication that the organisms responded to a mutation by attempting to eliminate it. A good example of this is the black dots that were spread throughout the cell. This is a potential mutation that was observed in just about all of the experimental cultures and its presence slowly declined throughout the generations. The researchers induced an environment that increased the amount of conjugation occurring. This is why it was so frequent. On a final note, it is interesting to once again revisit the fact that the Paramecia could often be found barreling through what was potentially concentrated cigarette toxin. This may have been an indication that some of the cells were actually addicted to the chemicals, much like how people become addicted to cigarettes. Potential follow up experiments include ones that use different concentrations of the cigarette toxin for varying periods of time. For example, rather than keeping the Paramecia in a twenty percent cigarette toxin solution for approximately three days, what if they were set in a forty percent solution for only one and a half. It would be interesting to see how the alterations to the organisms differ with such changes. One could also try to isolate different toxins within the cigarettes and see how they individually affect the Paramecia. Different brands of cigarettes could be tested too to see the differences between the effects caused by each. These are just a few of the many examples of follow-up experiments that could be performed. Work Cited E u k a r y o t es g e n o me s - p a r a m e c i u m t e tr a u r el i a . ( n . d . ) . R e t r i ev e d f r o m h t t p : / / ww w .e b i . a c . u k/ 2 c a n/ g e no m e s / e u k ar y o te s/ P a r am e c i u m _ te t r a u r e l i a . h tm l K l e b s i e l l a p n e u m o n i ae . ( n . d . ) . R e t r ie v e d f ro m h t t p : / / ww w . p h a g e t her a p y c e n te r . c o m / p i i / P a t i e n t S e rv l e t? co m m an d = s t a t i c _k l e b s i e l l a P a r a me c i u m te t r a ur e li a . ( 2 0 1 1 , A u g u s t 0 9 ) . R e t r ie v e d f r o m h t t p : / / ww w .m et a m ic ro b e . co m / p a r am e c i um /