Water Properties (10-8-13) -cp
advertisement

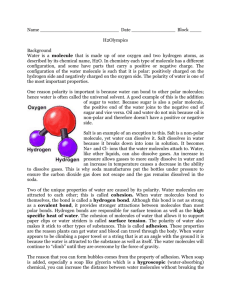
Water Properties 10/6/13 Materials: -water -food coloring -paper towels -plastic cups -celery -pepper -dish soap -pennies Opening Discussion -Why is water so important to life? -Examples of where we find/use water? (in the body, hydration, rain for plants, oceans, cools earth’s atmosphere, ect.,) -Properties of water: -Cohesion: water molecules sticking to eachother -Adhesion: water molecules sticking to other molecules - Capillary Action: ability of liquid to travel upward in a narrow space, acting against gravity -Surface Tension: strong cohesion of water molecules on surface creates aids to resist external forces Experiment #1 (Capillary action) Demonstrate the effects of capillary action by setting up three cups, one with blue colored water, one with red colored water, and one empty cup in the middle. Make simple paper towel bridges to connect the three cups and allow the apparatus to sit while doing the other experiments. (capillary action will allow the red and blue water to travel into the empty cup and turn purple) Return to this experiment at the end of the class period and discuss Experiment #2 (Capillary action) Give each student a cup, have them label it, add water and food coloring of their choice, give them each a small stalk of celery and allow it to sit until next week. The celery will soak up the food coloring via capillary action and change color. Experiment #3 (surface tension) -sprinkle pepper on the surface of water (either in individual cups for the kids or in a pie tin for demonstration) -dip finger in dish soap and touch surface of the water (the molecular chemistry of the soap causes it to break the surface tension of the water forcing the pepper to the outsides of the surface, away from the soap where there is still some surface tension present) Experiment #4 (Surface tension, cohesion and adhesion) -give each students a paper towel, a penny and a plastic pipette -challenge them to use the pipette to collect as many drops of water on the penny as possible -see who can get the most drops on the penny without the water spilling over (this is a result of cohesive forces and surface tension that forms water dome on the penny) -swap out their water for soapy water and challenge them to get as many soapy water drops on the penny as possible (the soap molecules in the water adhere to the water molecules preventing a lot of cohesion, thus making it more difficult to add drops of water onto the penny) Remember to revisit experiment #1 Conclusion Discussion: -what did we learn? -what property of water allows plants to get their water? (capillary action) -what property of water allows water bugs to “skate” on water? (surface tension) -what property of water allowed us to make a water dome on the penny? (cohesion) -what property of water prevented the dome from being made when we added soap? (adhesion) -other reason why water is important?