Laboratory Inspection Checklist
advertisement

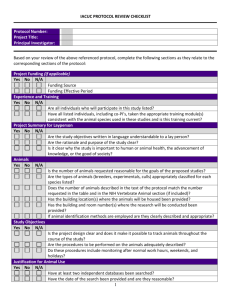
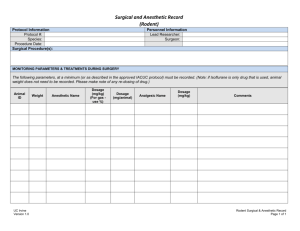
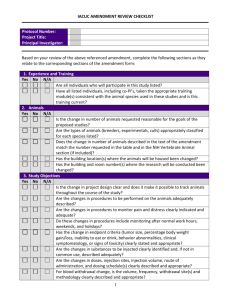
Institutional Animal Care and Use Committee University of California-Davis Animal Study Areas Inspection Checklist (Procedure Areas: Minor and Non-Survival Surgeries, Laboratories and Rodent Surgeries) Date:_____________________ Building/room: *Routine procedures: Species: *Surgical procedures: P.I.: Survival? Terminal? Multiple Survival Surgery? Department: Designated Contact: Reviewed by: Phone: A. PROGRAMMATIC Staff in attendance: *** A M S 1. Protocol(s) available , # PPM 290-30 Protocol status sheet (review) Protocol staff roster (review) 2. Occupational Health (Physical) –PPE, hand wash, lab coats PPM 290-25 3. Occupational Health (Preventative Med) - questionnaire 4. Training records 5. Fish and Game Permit 31. Animal Tracking System B. GENERAL CONCERNS 6. 7. 8. 9. 10. 11. 12. 2011 ILAR Guide reference pages Animal housing, food and water provisions (if more than 6 hours) 63-71 Sanitation of procedure area 72-73 Carcass and waste disposal 73-74 Security 151 Animal Identification 75 Vet care 105-114 Drugs/expiration dates/Pharmaceutical grade 31 13. Transportation of animals to the study area. 107-109 14. Euthanasia methods appropriate 2007 AVMA Panel/Guide 123 15. Sharps disposal UCD Safety Net #3, 62 30. Are personnel trained on how to report a concern? C. SURVIVAL SURGERY (rodent or minor procedures only) 2011 ILAR Guide pg. 115-120 [if yes, complete] 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. Rodent survival surgery clean and uncluttered Records of (pre, peri, and post-operative) Aseptic procedures Autoclave monitoring procedures Storage of autoclaved materials Cold sterilization procedures Anesthetic monitoring (including, maintenance of vaporizer) Gas cylinders immobilized Scavenging of anesthetic gases Analgesics used according to protocol * Surgery - any minor, non-survival surgery or rodent surgery ** Routine procedure - any non-surgical procedure (e.g. injections, stomach tubing, where animals are taken to the lab, etc.) ***A=acceptable; M=minor; S=significant deficiency (is or may be a threat to animal health or safety); fol/up=follow-up Study area inspection checklist Revised April 20, 2011 n/a Fol/up Institutional Animal Care and Use Committee University of California-Davis Building/room: COMMENTS: Unless otherwise noted, correction of all “Minor Deficiencies” identified must occur within 30 days from the date of inspection. Alternate dates may be negotiated at the time of the inspection, but must be identified in writing. If “Significant Deficiencies” are noted, corrective actions are expected to be implemented immediately, with final correction dates determined by the UC Davis IACUC. CC: IACUC PI Designated Contact Laboratory Inspection File Study area inspection checklist Revised April 20, 2011