Instructions for Equilibrium Lab
advertisement

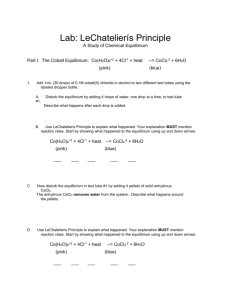
Instructions for Equilibrium Lab For Part 1 Because you added a large amount of SCN-, and dilute Fe+3, essentially all of the Fe+3 are converted to FeSCN+2. Therefore, [Fe+3] = [FeSCN+2] Plot absorbance on y-axis, and [FeSCN+2] on x-axis. Use a best fit line! Do not connect the dots! For Part 2 You can determine the [FeSCN+2] at equilibrium by using the graph from part 1, Absorbance measured corresponds with [FeSCN+2] at equilibrium. Fill in table #2. For Part 3 Determine initial concentrations of Fe+3 and SCNUse ICE to determine K Change in x will be the same as the concentration of [FeSCN+2] at equilibrium.