January 14, 2013
advertisement
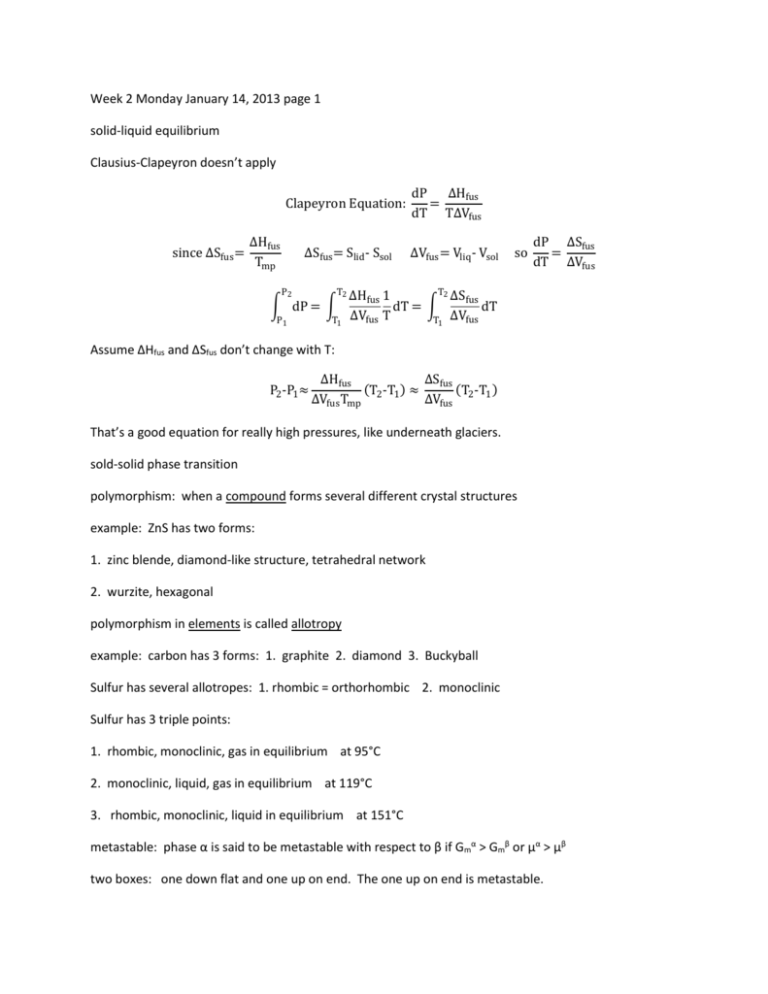
Week 2 Monday January 14, 2013 page 1 solid-liquid equilibrium Clausius-Clapeyron doesn’t apply Clapeyron Equation: since ∆Sfus = ∆Hfus Tmp ∆Sfus = Slid - Ssol P2 T2 ∫ dP = ∫ P1 T1 dP ∆Hfus = dT T∆Vfus ∆Vfus = Vliq - Vsol so dP ∆Sfus = dT ∆Vfus T2 ∆Hfus 1 ∆Sfus dT = ∫ dT ∆Vfus T T1 ∆Vfus Assume ΔHfus and ΔSfus don’t change with T: P2 -P1 ≈ ∆Hfus ∆Sfus (T2 -T1 ) ≈ (T -T ) ∆Vfus Tmp ∆Vfus 2 1 That’s a good equation for really high pressures, like underneath glaciers. sold-solid phase transition polymorphism: when a compound forms several different crystal structures example: ZnS has two forms: 1. zinc blende, diamond-like structure, tetrahedral network 2. wurzite, hexagonal polymorphism in elements is called allotropy example: carbon has 3 forms: 1. graphite 2. diamond 3. Buckyball Sulfur has several allotropes: 1. rhombic = orthorhombic 2. monoclinic Sulfur has 3 triple points: 1. rhombic, monoclinic, gas in equilibrium at 95°C 2. monoclinic, liquid, gas in equilibrium at 119°C 3. rhombic, monoclinic, liquid in equilibrium at 151°C metastable: phase α is said to be metastable with respect to β if Gmα > Gmβ or µα > µβ two boxes: one down flat and one up on end. The one up on end is metastable. examples of metastable: diamond, superheated H2O(liq), supercooled liquid, supersaturated solution, superheated water coming out of undersea geysers higher order transitions - everything we’ve done so far is first-order liquid-gas or solid-liquid: Δ = qp ≠ 0 ΔV ≠ 0 1st order ∂H CP = ( ) ∂T P CP at mp (melting point) is not defined higher order transitions For second order, CP increases by a finite amount. CP = ( ∂H ) does not apply to higher order transition ∂T P ΔV = 0 ΔH = qP = 0 Example of second order transition: normal conductivity to superconductivity For Hg: Ttran = 4.2K at 1atm Clausius-Clapeyron is meaningless, since ΔV = 0 For lambda (λ) transitions, CP approaches ∞ from both sides examples of lambda transitions: paramagnetism to ferromagnetism liquid He(I) to liquid He(II)











