Appendix 13 Balancing Equations Assignment
advertisement
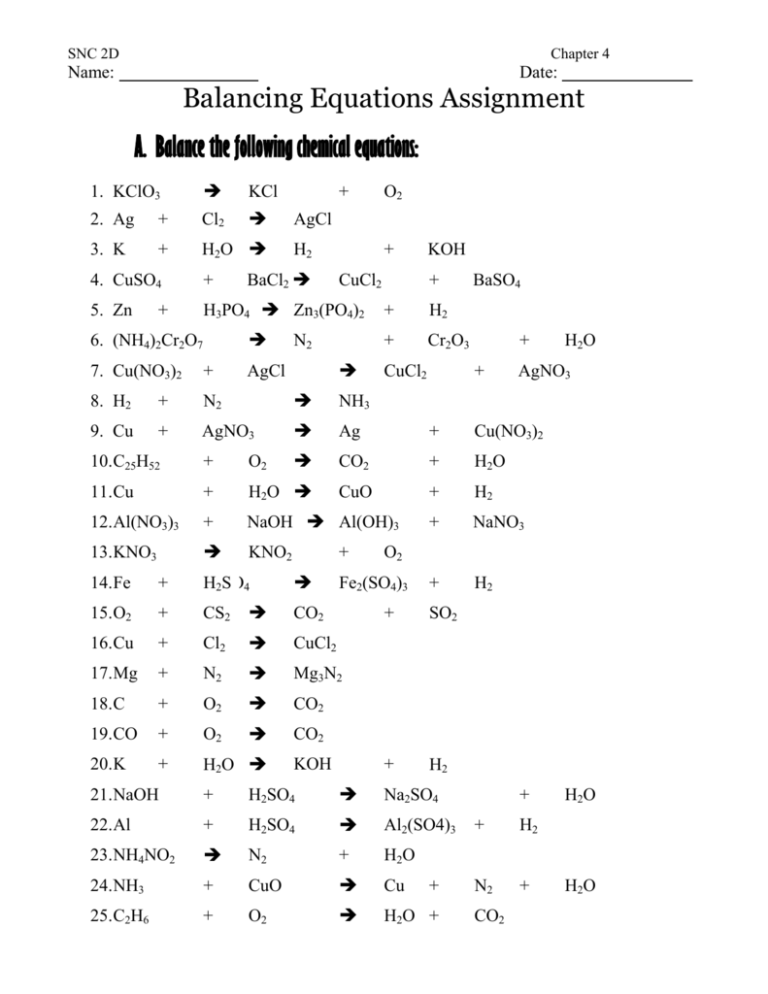
SNC 2D Chapter 4 Name: Date: Balancing Equations Assignment A. Balance the following chemical equations: 1. KClO3 KCl 2. Ag + Cl2 3. K + H2O H2 + O2 AgCl + BaCl2 CuCl2 KOH 4. CuSO4 + 5. Zn H3PO4 Zn3(PO4)2 + H2 N2 + Cr2O3 + 6. (NH4)2Cr2O7 + CuCl2 AgCl BaSO4 + 7. Cu(NO3)2 + 8. H2 + N2 NH3 9. Cu + AgNO3 Ag + Cu(NO3)2 10.C25H52 + O2 CO2 + H2O 11.Cu + H2O CuO + H2 12.Al(NO3)3 + NaOH Al(OH)3 + NaNO3 13.KNO3 KNO2 + H2 + + H2SO4 15.O2 + CS2 CO2 16.Cu + Cl2 CuCl2 17.Mg + N2 Mg3N2 18.C + O2 CO2 19.CO + O2 CO2 20.K + H2O KOH AgNO3 O2 Fe2(SO4)3 14.Fe + H2O + SO2 + H2 21.NaOH + H2SO4 Na2SO4 22.Al + H2SO4 Al2(SO4)3 23.NH4NO2 N2 + 24.NH3 + CuO Cu 25.C2H6 + O2 H2O + + + H2 N2 + H2O H2O + CO2 H2O SNC 2D Chapter 4 26.P4O10 + H2O H3PO4 27.SO2 + O2 SO3 28.CH4 + O2 CO2 + H2O 29.AlCl3 + K KCl + Al B. Write the formula for each word equation, and then write the balanced formula: 1. sodium hydroxide 2. iron + sodium oxide oxygen + water iron (II) oxide 3. iron (III) sulfide + hydrogen chloride iron (III) chloride + hydrogen sulfide 4. aluminum nitrate + sulfuric acid aluminum sulfate + nitric acid 5. barium + water hydrogen 6. calcium + oxygen (gas) calcium oxide 7. sulfur dioxide 8. phosphoric acid + + barium hydroxide oxygen (gas) sulfur trioxide + sodium hydroxide water + 9. copper (II) oxide + hydrogen (gas) copper 10.magnesium + sodium phosphate + sulfuric acid magnesium sulfate + 11.lead (II) nitrate + potassium iodide lead (II) iodide + water hydrogen (gas) potassium nitrate