Sample Paper - Red Remedia
advertisement

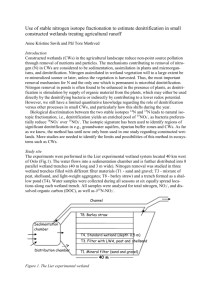
RELATIVE ABUNDANCE OF DENITRIFICATION GENES AND N2O EMISSIONS IN TWO CONSTRUCTED WETLANDS IN DOÑANA NATURAL SPACE Correa-Galeote D., Tortosa G., Bedmar E. J. Department of Soil Microbiology and Symbiotic Systems, Estación Experimental del Zaidín, Agencia CSIC, 18008 Granada, Spain 1. Introduction In the biogeochemical N cycle, denitrification is the process by which nitrate (NO3-) is reduced to dinitrogen gas (N2) via nitrite (NO2-), nitric oxide (NO) and nitrous oxide (N2O) when oxygen concentration becomes limiting. This process is carried out by the sequential activity of the enzymes nitrate reductase (Nar/Nap), nitrite reductase (NirK/NirS), nitric oxide reductase (Nor) and nitrous oxide reductase (NosZ), encoded by the narG/napA, nirK/nirS, norC and nosZ genes. Incomplete denitrification, however, results in greenhouse gases (NO and N2O) release into the atmosphere, thus contributing to global climatic change. Previous work has shown differences in nitrate concentration along la Rocina stream within Doñana Natural Space (DNS), resulting in variations in greenhouse gases production by the sediments (Tortosa et al. 2011). The spatial distribution of denitrifying microbial populations and their associated N2O production have been described for los Guayules, a constructed wetland within DNS (Correa-Galeote et al. 2012). However, abundance of denitrification genes in relation with nitrate contamination in Doñanas’s constructed wetlands has not been published. The goal of this work is a) to analyze the abundance of denitrification genes in los Guayules and los Mimbrales constructed wetlands, b) to determine activity of denitrifying bacterial populations, c) to analyze correlations between denitrification genes and their function, and d) to use the obtained data to propose a denitrification gene as a molecular marker for estimation of the denitrification process in environmental samples. 2. Materials and methods Two wetlands, los Mimbrales (W1, UTM coordinates 29S 0721735, 4108590) and los Guayules (W2, UTM coordinates 29S 0722249, 41094599) located in DNS were used in this study. Four independent samples within 1 m2 of the sediments from each wetland were taken in April and October, representing the wet and dry season, respectively, of years 2009 and 2010. Nitrate was estimated by HPLC as indicated by Tortosa et al. (2011). DNA was extracted from the sediments as previously published (Correa-Galeote et al. 2012). Abundance of the denitrifying community was estimated by quantification of the narG, napA, nirK, nirS and nosZ genes by real-time PCR (qPCR) as described earlier (Correa-Galeote et al. 2012). Relative abundance was calculated as the ratio between each denitrification gene abundance and the 16S rRNA gene abundance. Denitrifying enzyme activity (DEA) of the sediments was measured as N2O production according to Tortosa et al. (2011). Because of the absence of homoscedasticity, the non-parametric MannWhitney (MWT) and Spearman tests were used for statistical analysis. 3. Results and discussion Nitrate concentration in sediments was statically higher (α = 0.05) in April than in October for each W1 and W2 wetlands, and each 2009 and 2010 years (Figure 1A). Nitrate content in W1 was lower that in W2 for each sampling season and each year 2009 and 2010 (Figure 1A). For each year 2009 and 2010, differences in the abundance of the denitrification genes were not found both between the two wetlands and between dry and wet seasons (data not shown). Despite differences in nitrate concentration, a comparison of the relative abundance of the narG, napA, nirK, nirS and nosZ genes in samples from W1 and W2 taken either in April or October 2009 showed no significant differences (data not shown). Similar results were obtained for year 2010. However, for each W1 and W2 wetlands, relative abundances of the napA, nirS and nosZ denitrification genes were significantly higher in April than in October regardless of the year (Table 1). This behavior was not observed for the narG and nirK genes (data not shown). A 160 B DEA (ng N-N2O g-1 h-1) mg NO3-/Kg sediment 140 120 100 80 60 40 20 0 W1 W1 W1 W1 April 09 Oct 09 April 10 Oct 10 W2 W2 W2 W2 April 09 Oct 09 April 10 Oct 10 350 300 250 200 150 100 50 0 W1 W1 W1 W1 April 09 Oct 09 April 10 Oct 10 W2 W2 W2 W2 April 09 Oct 09 April 10 Oct 10 Figure 1. Nitrate concentration (A) and denitrifying enzyme activity (DEA) (B), in sediments from two constructed wetlands in DNS. W1: Los Mimbrales, W2: Los Guayules. Values of DEA were always higher in April for each W1 and W2 wetlands and years 2009 and 2010 (Figure 1B). When denitrification activity was compared between the two wetlands, W1 had lower values than W2, regardless of the year (Figure 1B). A correlation among nitrate concentration, DEA and gene relative abundances showed that maximal correlation was found between the nosZ gene and DEA, with a ρ value of 0.618. Table 1. Relative abundance of the denitrification genes napA, nirS and nosZ in sediments from two constructed wetlands in DNS. W1: Los Mimbrales, W2: Los Guayules. Values represent the mean ± standard error (n = 4). Year Sampling month April 2009 October April 2010 October Sampling site W1 W2 W1 W2 W1 W2 W1 W2 Relative abundance of denitrification genes (%) napA nirS nosZ 9.76 ± 0.33 6.53 ± 0.47 0.50 ± 0.01 15.05 ± 1.56 5.71 ± 0.20 0.49 ± 0.01 6.28 ± 0.39 3.68 ± 0.15 0.41 ± 0.01 4.43 ± 0.20 3.14 ± 0.07 0.38 ± 0.01 9.97 ± 0.36 4.64 ± 0.21 0.54 ± 0.02 10.78 ± 0.24 6.17 ± 0.25 0.51 ± 0.01 6.54 ± 0.24 3.69 ± 0.19 0.31 ± 0.01 5.66 ± 0.18 3.77 ± 0.23 0.31 ± 0.01 4. Conclusions 1. The relative abundance of denitrification genes (napA, nirS and nosZ) is affected by the dry and wet seasonal variations. 2. Nitrous oxide production in constructed wetlands is dependent upon nitrate concentration and the relative abundance of the denitrification genes. 3. The nosZ gene is proposed as a molecular marker for estimation of denitrification activity in constructed wetlands. 5. References Correa-Galeote D., Marco D.E., Tortosa G., Bru D., Philippot L., and Bedmar E.J. 2012. Spatial distribution of N-cycling microbial communities showed complex patterns in constructed wetland sediments. FEMS Microbiology Ecology DOI: 10.1111/j.1574-6941.2012.01479.x. Tortosa G., Correa D., Sánchez-Raya A.J., Delgado A., Sánchez-Monedero M.A., and Bedmar E.J. 2011. Nitrate contamination, biogeochemical properties and biological activities in surface waters and sediments of La Rocina stream (Doñana National Park, SW Spain): Greenhouse gas emissions and denitrification. Ecological Engineering 37: 539-548. This study was supported by ERDF-cofinanced grant RNM4746 from Consejería de Economía, Innovación y Ciencia (Junta de Andalucía, Spain).