Vibronic Spectrum of Permanganate Ion Lab
advertisement

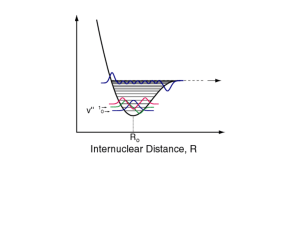
Vibronic Spectrum of the Permanganate Ion Feb 24th, 2012 1 Prelab calculation Determined an aliquot of your potassium permanganate solution required to make 100 mL of a 10-3 mol/L solution. 2 Background In solution UV-visible spectroscopy, it is normally not possible to observe vibrational transitions for two of reasons: 1 2 Electronic, vibrational and rotational transitions are occurring simultaneously. There is exchange of energy with translational modes which broadens the bands. The permanganate anion provides a stunning exception to this rule. In this species, provided we do the experiment carefully, we can directly observe vibronic (vibrational and electronic) transitions. The initial population in excited vibrational states is very small. Accordingly, we observe transitions from the ground electronic state with the vibrational quantum number n = 0. The end states have different amounts of vibrational excitation. The vibrational frequency depends on the electronic state so, if the electronic quantum number is Λ, we can think of the energy of the molecule as being given by an equation of the form EΛ,n = Z(Λ) + ħω0(Λ) (n + ½) + (small terms) where Z(Λ) is the zero-point energy of the electronic state Λ. The small terms include rotational terms as well as anharmonic contributions. During a vibronic transition, the energy gained by absorption of a photon is therefore ΔE(Λf ,nf )=ΔZ +ħω0(Λf ) (nf +½ ) - ½ ħω0 (Λ0) where Λ0 is the ground-state electronic quantum number and Λf and nf are the final (excited state) values of these quantum numbers. Now consider two vibronic transitions whose nf values differ by 1. Then Δ(ΔE) = ΔE(Λf ,nf + 1) - ΔE(Λf ,nf ) = ħω0(Λf ). (1) Thus the vibronic band differ in photon frequency by the fundamental vibrational frequency of the electronically excited state. 3 Safety Potassium permanganate is a strong oxidizing agent. 4 Procedure Prepare a 10-3 mol/L solution of potassium permanganate. Fill a cuvette with your solution and obtain a spectrum in the wavelength range 450–600 nm. You must use a narrow beam 1 width (0.2 nm) and a slow scanning speed (20 nm/min). 5 Reporting You should observe at least three maxima. Convert the wavelengths of these maxima to angular frequencies. Subtract the frequencies of adjacent maxima. According to eqn 1, the differences should be ω0(Λf ), the fundamental vibrational frequency of the excited electronic state. The permanganate anion is tetrahedral. Accordingly, it has nine normal modes of vibration. It turns out that the vibrational mode observed in this experiment is the totally 2 symmetric stretch. For totally symmetric stretches, ____ ω0 =√ k/mL 16 3 where mL is the mass of one of the ligand atoms, O in this case. Compute k(Λf ), the bond stiffness of the Mn-O bond in the excited electronic state. Because of the selection rules, the totally symmetric stretch does not appear in the normal IR spectrum. However, it can be detected by Raman spectroscopy. In the electronic 4 ground state, the totally symmetric stretch has a frequency of 844 cm-1. Compute the Mn-O bond stiffness in the ground electronic state of the ion. 1 If the spectrometer you are using allows you to set the signal averaging time and data interval, set these to 0.6 s and 0.2 nm, respectively. 2 A.B.P. Lever, Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, 1984; p 323. 3 16 Your sample is not isotopically pure, but the natural abundance of O is 99.759%. 4 S.J.A. Pope and Y.D. West, Spectrochim. Acta A, 51, 2027–2037 (1995). Marc R. Roussel