July 2015 - Positive Recommendations (Word 87KB)
advertisement

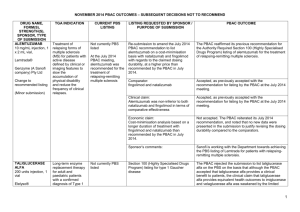
JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION ACICLOVIR 30mg/g (3%) eye ointment, 4.5g DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Herpes simplex keratitis Temporary Restricted Benefit listing of as an alternative to currently listed aciclovir (Zovirax). The PBAC recommend the temporary Restricted Benefit listing of aciclovir (AciVision) on the PBS as an alternative to currently listed but unavailable, aciclovir (Zovirax), to ensure access to a treatment for Herpes simplex keratitis. Chronic obstructive pulmonary disease Authority Required (STREAMLINED) listing for the treatment of patients with chronic obstructive pulmonary disease. The PBAC recommended the listing of aclidinium/eformoterol fixed dose combination (FDC) as an Authority required (STREAMLINED) benefit for the treatment of chronic obstructive pulmonary disease for patients already stabilised on concomitant long acting muscarinic receptor antagonist (LAMA) and long-acting selective β2 agonist (LABA) therapy. ACIVISION® Medsurge Healthcare Pty Ltd New listing (Minor Submission) ACLIDINIUM BROMIDE with EFORMOTEROL FUMARATE DIHYDRATE aclidinium 340 microgram/actuation + eformoterol 12 microgram/actuation inhalation: powder for, 60 actuations BRIMICA® GENUAIR® A.Menarini Australia Pty Ltd The PBAC recommended the listing on a cost-minimisation basis to the existing LAMA/LABA fixed dose combinations, umeclidinium/vilanterol and glycopyrronium/indacaterol. The equi-effective doses are considered to be aclidinium 340 microgram with eformoterol 12 microgram (twice daily), umeclidinium 62.5 microgram with vilanterol 25 microgram (daily), and glycopyrronium 50 microgram with indacaterol 110 microgram (daily). New listing (Major Submission) 1 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION ANAKINRA anakinra 100 mg/0.67 mL injection, 28 x 0.67 mL syringes DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION Cryopyrin-associated periodic syndromes Correspondence from the Australasian Society of Clinical Immunology and Allergy requested that immunologists be granted prescribing rights for anakinra for the treatment of cryopyrinassociated periodic syndromes. The PBAC recommended that the treatment criteria for this indication should be modified to include prescribing by clinical immunologists. Non-Hodgkin's lymphoma Re-submission for Section 100 (Efficient Funding of Chemotherapy arrangements) listing for the first line treatment of indolent non-Hodgkin's lymphoma (iNHL) and mantle cell lymphoma (MCL). The PBAC recommended the listing of bendamustine for the treatment of iNHL and MCL. The PBAC considered that bendamustine presented a less toxic alternative to existing treatments for some patients with iNHL and MCL and accepted that it improved progression free survival. The PBAC also considered that listing of bendamustine on the PBS may potentially result in cost savings to the Commonwealth. KINERET® A. Menarini Australia Pty Ltd Change to listing PBAC OUTCOME (Correspondence) BENDAMUSTINE powder for injection 100 mg vial, 1 powder for injection 25 mg vial, 1 Ribomustin® Jansen-Cilag Pty Ltd New listing (Minor Submission) 2 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION BOSENTAN; EPOPROSTENOL; MACITENTAN bosentan 62.5 mg tablet, 60; bosentan 125 mg tablet, 60; epoprostenol 500 microgram injection vial, 1; epoprostenol 1.5 mg injection vial, 1; macitentan 10 mg tablet, 30 DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Pulmonary Arterial Hypertension (PAH) Removal of the following requirements from the 'Continuing treatment' restriction: (i) patient must have been assessed as having achieved a response to their most recent course of treatment; and (ii) clinicians must provide evidence that a patient has been assessed as having achieved response when wanting to prescribe continued treatment. The PBAC considered that progression of disease in patients on PAH agents does not necessarily indicate that the therapy is no longer effective and that the biggest response will most likely be achieved in response to the initial treatment. The PBAC therefore agreed that it would be reasonable to have a first continuing treatment restriction where evidence of a response to the PAH agent must be demonstrated; and then a subsequent continuing treatment restriction, which would not require further evidence of a response, i.e. removal of the requirement for a patient to have been assessed as having achieved a response to their most recent course of treatment, and thereby, the removal of the requirement for clinicians to provide results from right heart catheterisation omposite assessment, echocardiography composite assessment and 6 minute walk test. Elevated intra-ocular pressure Restricted Benefit listing for the treatment of elevated ocular pressure in adult patients with open-angle glaucoma or ocular hypertension. The PBAC recommended the Restricted Benefit listing of brinzolamide + brimonidine FDC for the treatment of elevated intra-ocular pressure on a cost-minimisation basis against a mixed comparator of dorzolamide + timolol FDC and the individual components of brinzolamide and brimonidine. TRACLEER®; VELETR®; OPSUMIT® Actelion Pharmaceuticals Australia Pty Ltd Change to listing (Minor Submission) BRINZOLOMIDE / BRIMONIDINE TARTRATE suspension containing brinzolamide 10 mg/mL + brimonidine 2 mg/mL, 5 mL Simbrinza® Alcon Laboratories Australia Pty Ltd New listing (Major Submission) 3 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION DAPAGLIFLOZIN and METFORMIN XR dapagliflozin 10 mg + metformin hydrochloride 500 mg tablet: modified release, 28 dapagliflozin 10 mg + metformin hydrochloride 1000 mg tablet: modified release, 28 dapagliflozin 5 mg + metformin hydrochloride 1000 mg tablet: modified release, 56 DRUG TYPE OR USE Type 2 diabetes mellitus LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Authority Required (STREAMLINED) listing for the treatment of type 2 diabetes as: 1) Triple oral therapy with metformin and a sulfonylurea; and 2) Add-on to insulin therapy. The PBAC recommended that the recommended listing of dapagliflozin+metformin XR for the treatment of type 2 diabetes mellitus be extended to include use in the add on to insulin and triple oral therapy settings, consistent with the current listings for dapagliflozin. XIGDUO® XR AstraZeneca Pty Ltd Change to recommended listing (Minor Submission) 4 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION DEFERASIROX 125 mg dispersible tablet, 28 250 mg dispersible tablet, 28 500 mg dispersible tablet, 28 DRUG TYPE OR USE Chronic iron overload LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION Section 100 (Highly Specialised Drugs Program) Authority Required listing for the treatment of chronic iron overload. PBAC OUTCOME The PBAC recommended revising the PBS restriction for deferasirox to an Authority required Section 100 listing for patients with: - transfusion dependent non-malignant disorders of erythropoiesis; - non-transfusion dependent thalassemias; and - transfusion-dependent malignant disorders with a median life expectancy greater than five years. EXJADE® Novartis Pharmaceuticals Australia Pty Limited Change to recommended listing (Major Submission) The PBAC rejected the request to retain the current PBS restriction for deferasirox for patients with “chronic iron overload in patients with disorders of erythropoiesis” as this very broad restriction enabled major use outside populations where cost-effectiveness has been demonstrated, in particular myelodysplastic syndrome. The PBAC did not accept that a survival benefit due to deferasirox therapy had been proved in myelodysplastic syndrome or other malignant disorders and therefore the cost-effectiveness of deferasirox in myelodysplastic syndrome generally was not adequately demonstrated. However, the PBAC recognised that iron overload is a cause of major morbidity and mortality in patients with longer survival, such as those with nontransfusion dependent thalassemias, and transfusion-dependent malignant disorders with a median life expectancy greater than five years. Under the restriction recommended by PBAC in November 2014, these patients could be significantly disadvantaged. Thus, the PBAC considered, subject to price negotiation, a revision to the restriction would be acceptable to allow use in these patient groups, noting the uncertainty of cost-effectiveness even in these subgroups. 5 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION EXENATIDE 2 mg, powder for injection, vial BYDUREON® AstraZeneca Pty Ltd Change to recommended listing (Major Submission) DRUG TYPE OR USE Type 2 diabetes mellitus LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Re-submission for Authority Required (STREAMLINED) listing for the treatment of type 2 diabetes mellitus as: 1) Dual combination therapy with metformin or a sulfonylurea; and 2) Triple combination therapy with metformin and a sulfonylurea; in a patient who meets certain criteria. The PBAC recalled that, in November 2013, it had recommended exenatide 2 mg once weekly as an Authority Required (STREAMLINED) benefit for dual combination therapy with metformin or a sulfonylurea and triple combination therapy with metformin and a sulfonylurea in patients with type 2 diabetes mellitus, on a cost-minimisation basis with exenatide 10 mcg twice daily (with a cost offset for reduced needle use). The PBAC considered that the simplified treatment regimen of reducing dosing frequency by 13 injections per week may translate into fulfilling an unmet need for high clinical need populations; specifically Indigenous, aged and mental health patients. The PBAC re-endorsed its November 2013 recommendation (of cost minimisation with a partial cost offset for reduced needle use) and recommended a further small price advantage for exenatide 2 mg once weekly on the basis of potential health benefits from likely improved adherence by a small number of high clinical need populations. 6 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION FENTANYL CITRATE (SUBLINGUAL) 100 microgram tablet: sublingual, 10 & 30, 200 microgram tablet: sublingual, 10 & 30, 300 microgram tablet: sublingual, 10 & 30, 400 microgram tablet: sublingual, 10 & 30, 600 microgram tablet: sublingual, 10 & 30, 800 microgram tablet: sublingual, 10 & 30, DRUG TYPE OR USE Breakthrough pain LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Re-submission for Authority Required (Palliative Care Schedule) listing for the treatment of breakthrough cancer pain. The PBAC recommended listing fentanyl citrate sublingual tablets as an Authority Required benefit on the Palliative Care Schedule for the treatment of breakthrough pain in patients undergoing palliative care for cancer. Restricted Benefit listing of an alternative medicinal food for patients with tyrosinaemia. The PBAC recommended the restricted benefit listing of glycomacropeptide and essential amino acids with vitamins and minerals, Tylactin® RTD 15 for the treatment of tyrosinaemia on a cost-minimisation basis against TYR Cooler® 20 and TYR Cooler® 15 on an equivalent price per gram of protein basis. The recommendation was made on a cost-minimisation basis with immediate-release opioids and fentanyl lozenge (Actiq). Equi-effective doses were not estimated, but assumptions were made for each pain episode to be treated with one sublingual tablet or one lozenge. The PBAC considered that the resubmission’s request of flat pricing across dose strengths addressed the issue of variability during titration of short acting fentanyl for breakthrough pain. ABSTRAL® A.Menarini Australia Pty Ltd New listing (Major Submission) GLYCOMACROPEPTIDE and ESSENTIAL AMINO ACIDS with VITAMINS and MINERALS 15 g protein oral liquid, 30 x 250 mL tetra pack Medicinal food TYLACTIN® RTD Cortex Health Pty Ltd New listing (Minor Submission) 7 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION INFLIXIMAB 100 mg injection vial, 1 100 mg injection vial, 3 100 mg injection vial, 4 INFLECTRA® Hospira Pty Ltd New listing (Minor Submission) DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Ankylosing spondylitis, Crohn's disease, Psoriatic arthritis, Rheumatoid Arthritis, Plaque psoriasis, Ulcerative colitis Section 100 (Highly Specialised Drugs Program) Authority Required listing of Inflectra (infliximab), a similar biological medicinal product, with the same indications and restrictions as the currently PBS listed brand of infliximab (Remicade®). The PBAC recommended the listing of infliximab (Inflectra) as a biosimilar of infliximab (Remicade), on a cost-minimisation basis to infliximab (Remicade), where the equi-effective doses are 100 mg infliximab (Inflectra) and 100 mg infliximab (Remicade). The PBAC recommended that the same indications that apply to infliximab (Remicade) should apply to infliximab (Inflectra). In relation to the “a” flagging of the Remicade and Inflectra brands of infliximab, the PBAC considered a range of factors including: There is evidence from three randomised clinical trials in treatment-naïve patients initiating on either Inflectra or Remicade that support a finding that Inflectra has equivalent effectiveness and equivalent safety compared to Remicade. The key randomised clinical studies in rheumatoid arthritis and ankylosing spondylitis did not indicate differences in efficacy or safety of Inflectra compared with Remicade. The supportive evidence from a series of noncomparative studies in patients with Crohn’s disease or ulcerative colitis demonstrates treatment with Inflectra is also effective and safe in these conditions. The clinical data provided in the submission did not suggest that there were any identified populations where the risks of using the biosimilar product in place of the reference biologic are disproportionately high. In the two trials with extension studies (Study 1.1 and Study 3.1), switching from Remicade to Inflectra occurred, and the clinical evidence suggested no difference in efficacy, safety or immunogenicity between the biosimilar and reference biologic in these studies. The ACPM has declared Inflectra a biosimilar for Remicade. The ACPM was satisfied of the similar safety and efficacy of Inflectra and Remicade in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis, ulcerative colitis and Crohn’s disease. The PBAC advised the Minister that it considered the Remicade and Inflectra brands of infliximab could be marked as equivalent in the Schedule of Pharmaceutical Benefits (“a” flagged), for the purposes of substitution by the pharmacist at the point of dispensing. The PBAC noted that it had held a consumer hearing on biosimilars, and that the issues discussed at the hearing in relation to biosimilar substitution had been considered by the PBAC when making its recommendation. 8 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME The PBAC noted that there appeared a public misunderstanding about how substitution occurs, and noted that the substitution process allows for patient and prescriber choice and is not automatic. For any individual prescription, a prescriber may choose to not permit brand substitution. If on the other hand, substitution has been permitted by the prescriber, the patient may choose which brand they wish to receive from the pharmacist. The PBAC noted that in relation to concerns about traceability, that Medicare routinely collects information on the brand of drug that is dispensed for a patient. In relation to concerns about multiple switches of brands, the PBAC considered that this is unlikely to occur in practice because infliximab is administered via an infusion with most patients receiving all courses of treatment at a single centre, reducing the likelihood of switches between brands. The PBAC recommended that the Department develop an implementation strategy for infliximab for the PBAC’s review before an “a” flag can be included against the Inflectra and Remicade brands on the Schedule. This implementation strategy should include an education campaign designed to support and promote the use of biosimilars, improving awareness and confidence by both health professionals and consumers to choose these products. The PBAC reiterated its position that it would consider the marking of equivalent (i.e. “a” flagging) in the Schedule of biosimilar medicines with their reference medicine on a case by case basis, taking into account the evidence presented in each submission to list a biosimilar medicine. 9 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION INSULIN GLARGINE 300 unit/mL, 1 x 1.5 mL cartridge 300 unit/mL, 3 x 1.5 mL cartridge 300 unit/mL, 5 x 1.5 mL cartridge TOUJEO® DRUG TYPE OR USE Diabetes LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION Unrestricted benefit listing. PBAC OUTCOME The PBAC recommended insulin glargine U300 as an unrestricted benefit on the basis of cost-minimisation to insulin glargine U100 for both type 1 and type 2 diabetes mellitus. The PBAC rejected the claimed benefit in hypoglycaemic events and the modelled cost effectiveness in type 2 diabetes mellitus. The PBAC noted the supportive comments from consumers but considered there was low clinical need for this additional formulation of insulin glargine. Sanofi-aventis New listing (Major Submission) Type 1 diabetes mellitus: On the basis of one head-to-head trial (EDITION IV) presented in the submission, U300 appears to have the same effect on glycosylated haemoglobin and frequency of adverse events as U100 in the treatment of type 1 diabetes mellitus. Type 2 diabetes mellitus: On the basis of three head-to-head trials (EDITION I, II and III), U300 appears to have the same effect on glycosylated haemoglobin as U100 in the treatment of type 2 diabetes mellitus. The PBAC did not accept U300 to have superior comparative safety in terms of reduced risk of hypoglycaemia. Based on the acceptance of non-inferiority of both comparative clinical effectiveness and safety, the PBAC agreed with the following equi-effective doses for type 2 diabetes mellitus: EDITION I: 1U of U300 = 0.91U of U100 EDITION II: 1U of U300 = 0.90U of U100 EDITION III: 1U of U300 = 0.88U of U100 The PBAC noted the 28 day shelf life of the U300 and that the increase in the number of units per cartridge may result in increased wastage in a small group of patients. The PBAC considered a small reduction in the price of the U300 was appropriate to account for the potential wastage. 10 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION LEUPRORELIN 45 mg injection: modified release [1 x 45 mg syringe] (&) inert substance diluent [1 x 2 mL syringe], 1 DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Prostate cancer Authority Required (STREAMLINED) listing of an additional strength of leuprorelin for the treatment of locally advanced or metastatic carcinoma of the prostate. The PBAC recommended listing an additional strength (45 mg) of intramuscularly administered leuprorelin for the treatment of locally advanced (equivalent to stage C) or metastatic (equivalent to stage D) carcinoma of the prostate. The PBAC accepted that there was a clinical place for the additional strength and considered that it was acceptably similar to the 3- and 4-month formulations with regard to effectiveness and the adverse events profile. The PBAC noted that listing the 45 mg intramuscular formulation would result in savings to the PBS. Central precocious puberty (CPP) Amendment to the PBS restriction wording of Lucrin® Depot Paediatric to specify that the patient must have had onset of signs or symptoms of central precocious puberty prior to the age of 8 years (girls) or 9 years (boys). The PBAC recommended that the criterion pertaining to patient age in the initial treatment restriction for leuprorelin for the treatment of CPP, be amended as follows: “Patient must be aged 10 years or younger (girls) or 11 years or younger (boys) AND Patient must have had onset of signs or symptoms of central precocious puberty prior to the age of 8 years (girls) or 9 years (boys)”. The PBAC noted that there may be a very small number of patients who may not have initiated treatment before 8 years (girls) or 9 years (boys) despite onset of symptoms before 8 years (girls) or 9 years (boys), and therefore considered it may be reasonable to extend the PBS age limit for initiating leuprorelin therapy. LUCRIN® DEPOT 6 month PDS AbbVie Pty Ltd New listing (Minor Submission) LEUPRORELIN 30 mg injection: modified release [1 x 30 mg syringe] (&) inert substance diluent [1 x 2 mL syringe], 1 LUCRIN® DEPOT AbbVie Pty Ltd Change to listing (Minor Submission) 11 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION NADROPARIN CALCIUM pre-filled syringe, 1900, 2850, 3800, 5700, 7600, 9500, 11400, 15200, 19,000 IU anti-Xa/mL DRUG TYPE OR USE Prevention of deep vein thrombosis LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION Unrestricted benefit listing and restricted benefit listing for prevention of clotting during haemodialysis. Fraxiparine® and Fraxiparine Forte® PBAC OUTCOME The PBAC recommended the listing of nadroparin (as Fraxiparine and Fraxiparine Forte) as an Unrestricted Benefit and as a Restricted Benefit listing (as Fraxiparine) for haemodialysis on a cost-minimisation basis against enoxaparin. The equi-effective doses are nadroparin calcium 111.9 IU is equal to enoxaparin sodium 1 mg. The PBAC advised that this equi-effective dose calculation appropriately excludes doses for haemodialysis, noting that in Australian clinical practice unfractionated heparin may also be used in this setting instead of enoxaparin. The TGA approved indication for Fraxiparine (nadroparin) includes haemodialysis. Aspen Pharmacare Australia Pty Ltd New listing (Major Submission) PARITAPREVIR with RITONAVIR, OMBITASVIR and DASABUVIR PARITAPREVIR with RITONAVIR, OMBITASVIR, DASABUVIR and RIBAVIRIN paritaprevir 75 mg + ritonavir 50 mg + ombitasvir 12.5 mg tablet, 56, with dasabuvir 250 mg tablet, 56 paritaprevir 75 mg + ritonavir 50 mg + ombitasvir 12.5 mg tablet, 56, with dasabuvir 250 mg tablet, 56 and ribavirin 200 mg tablet, 168 paritaprevir 75 mg + ritonavir 50 mg + ombitasvir 12.5 mg tablet, 56, with dasabuvir 250 mg tablet, 56, and ribavirin 600 mg tablet, 56 VIEKIRA PAK® VIEKIRA PAK-RBV® AbbVie Pty Ltd Hepatitis C Authority Required (STREAMLINED) listing for the treatment of patients with genotype 1 chronic hepatitis C (CHC). The PBAC recommended the Authority Required listing of paritaprevir/ritonavir/ombitasvir plus dasabuvir (Viekira PAK) and paritaprevir/ritonavir/ombitasvir plus dasabuvir with ribavirin (Viekira PAK-RBV) for the treatment of patients with Genotype 1 chronic hepatitis C (CHC) infection on the basis of non-inferior efficacy and safety with Ledipasvir/Sofosbuvir (LDV/SOF), recommended in March 2015. The PBAC considered that it was reasonable to assume that one treatment course of Viekira PAK/Viekira PAK-RBV was as effective as one course of LDV/SOF. The PBAC reiterated that new treatments for CHC infection were very effective and listing of these products would offer options for treatment of Genotype 1 CHC. The PBAC reiterated that it was appropriate for the new all oral treatment to be listed in the General Schedule, rather than Section 100 Highly Specialised Drug Program, to facilitate the longer term objectives for access to treatment, increase treatment rates and better outcomes with a view to treat all patients with CHC over time. The PBAC did not accept that the treatments are cost-effective at the price proposed by the sponsor in the overall patient population with Genotype 1 CHC. 12 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME New listing The PBAC noted that there was a prevalent population of approximately 230,000 patients with CHC in Australia, of which approximately 50% have Genotype 1 CHC. The estimates of the number of patients treated with the availability of an all oral interferon free treatment, presented by the DUSC for the March 2015 PBAC meeting, indicated that approximately 62,000 patients with any Genotype CHC could be treated in the next years. The PBAC noted that at the price and patient estimates submitted by the sponsor, the proposed budget impact was less than $2 billion over 5 years for the patient population with Genotype 1 CHC. (Major submission) The PBAC confirmed their previous advice to the Minister: • that there is the high clinical need for all oral interferon-free treatments of CHC to be made available on the PBS, • that there was no basis on which to recommend that any one treatment be more expensive than another, • there is a large opportunity cost to health care system. Given this large opportunity cost, the cost of a course of treatment should be set irrespective of the duration, and that other pricing policies be considered. PERTUSSIS VACCINEACELLULAR, combined with DIPTHERIA and TETANUS TOXOIDS 0.5 ml vial, 1 TRIPACEL® Prevention of diphtheria, tetanus and pertussis National Immunisation Program (NIP) listing of an additional booster dose of DTaP vaccine for children aged 18 months. The PBAC recommended including the 18-month booster of the pertussis vaccine-acellular, combined with diphtheria and tetanus toxoids (Tripacel), on the NIP for the prevention of pertussis on the basis of cost-minimisation to the 18-month booster of the combined diphtheria, tetanus and acellular pertussis (Infanrix) vaccine recommended at the November 2014 PBAC meeting for inclusion on the NIP. Based on the evidence provided, the PBAC considered it would be reasonable to conclude that the two vaccines at the 18-month time point conferred similar efficacy and safety. Sanofi-aventis Australia Pty Ltd New listing (Major submission) 13 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION PNEUMOCOCCAL CONJUGATE VACCINE (13-valent) 0.5 mL injection PREVENAR 13® Pfizer Australia Pty Ltd DRUG TYPE OR USE Prevention of pneumococcal disease LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION Re-submission to extend the listing on the National Immunisation Program (NIP) to include the prevention of pneumococcal disease in non-Indigenous adults aged 65 years and Indigenous adults aged 50 years. PBAC OUTCOME The PBAC recommended including the 13-valent pneumococcal conjugate vaccine (13vPCV) on the NIP for the prevention of pneumococcal pneumonia and invasive pneumococcal disease in adults on the basis of cost-minimisation to the 23-valent pneumococcal polysaccharide vaccine (23vPPV). The PBAC recommended that a single dose of 13vPCV be made available to pneumococcal vaccine naïve non-Indigenous adults aged 65 years and over and to pneumococcal vaccine naïve Indigenous adults aged 50 years and over. Change to listing (Minor Submission) The PBAC noted that if 13vPCV were included on the NIP for adults, then individuals in specified at-risk groups would continue to receive a booster dose of 23vPPV five years following the primary dose of 13vPCV. 14 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION PONATINIB 15 mg tablet, 60 45 mg tablet, 30 ICLUSIG® Specialised Therapeutics Australia Pty Ltd New listing (Major submission) DRUG TYPE OR USE Chronic myeloid leukaemia (CML) and acute lymphoblastic leukaemia (ALL) LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION Re-submission for Authority Required listing for the treatment of CML or ALL in a patient who meets certain criteria. PBAC OUTCOME The PBAC recommended the PBS-listing of ponatinib as an Authority Required benefit for treatment of CML in (i) Patients who have failed first line therapy with imatinib or dasatinib or nilotinib and whose CML has the T315I mutation; (ii) Patients with CML where both nilotinib and dasatinib have failed or where one of nilotinib or dasatinib has failed and who are intolerant of the other drug; and for the treatment of relapsed or refractory Philadelphia chromosome positive (Ph+ ) ALL in patients whose ALL has the T315I mutation. The PBAC reaffirmed its previous conclusion that ponatinib is the most active tyrosine kinase inhibitor for CML patients who carry the T315I mutation, and that response rates to ponatinib in CML patients with the T315I mutant CML are similar to those seen in CML patients without this mutation treated with dasatinib or nilotinib in second line. The PBAC reaffirmed its view that ponatinib has an inferior toxicity profile to imatinib, dasatinib and nilotinib, especially with regard to serious vascular occlusive events. The PBAC considered that there was a clinical need for treatments of Ph+ ALL patients without the T315 mutation (including patients with less common mutations such as F317L and E225V). However, in the absence of additional evidence, the PBAC could not recommend approval for ponatinib in Ph+ ALL without the T315 mutation. The PBAC did not alter its view from November 2014 in recommending the use of ponatinib in clinical scenarios where its risk:benefit ratios were most favourable. The PBAC would welcome additional quality evidence of the benefit of pontantib in Ph+ ALL patients without the T315 mutation to facilitate any future reconsideration. The PBAC considered that the cost-effectiveness of ponatinib would be acceptable when benchmarked against the costs of dasatinib and nilotinib, with adjustments required to account for the toxicity of the treatments. The equi-effective doses were considered to be ponatinib 30.2 mg daily, dasatinib 102 mg daily and nilotinib 797 mg daily. 15 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION QUADRIVALENT INFLUENZA VACCINE 0.5 ml pre-filled syringe, 5; 0.5 ml pre-filled syringe, 10; 0.25 ml pre-filled syringe, 5; 0.25 ml pre-filled syringe, 10 DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Prevention of seasonal influenza National Immunisation Program (NIP) listing for the prevention of seasonal influenza in the currently eligible patient populations. The PBAC recommended the listing of the quadrivalent influenza vaccine, FluQuadri, on the NIP – Designated Vaccines List for the prevention of seasonal influenza. The recommendation was based on a cost-minimisation with Fluarix Tetra quadrivalent influenza (for individuals 3 years and above) and with trivalent influenza vaccine (for individuals less than 3 years). Severe active granulomatosis with polyangiitis and microscopic polyangiitis The March 2015 major submission sought Section 100 (Highly Specialised Drugs Program) Authority Required listing for rituximab for remission induction in patients with severe active granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). The PBAC recommended the listing of rituximab, on the basis that it should be available only under special arrangements under the Section 100 Highly Specialised Drugs Program, for remission induction in patients with severe active GPA and MPA. FLUQUADRI® Sanofi-aventis New listing (Major Submission) RITUXIMAB injection for infusion 500mg/50mL Mabthera® Roche Products Pty Ltd. (Deferral from March 2015 PBAC meeting) At its March 2015 meeting, the PBAC considered that there is a high clinical need for rituximab for rare and potentially life-threatening conditions such as GPA and MPA. The PBAC considered that the claim of non-inferior comparative effectiveness and safety of rituximab compared to cyclophosphamide made in the submission was reasonable. The PBAC requested the Department negotiate a Risk Share Arrangement in a manner that can be implemented and managed by the Department. 16 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION RIVASTIGMINE 13.3 mg/24 hours patch, 30 DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Dementia Authority Required listing of an additional strength for the treatment of mild to moderately severe Alzheimer-type dementia. The PBAC recommended the listing of rivastigmine for the treatment of mild to moderately severe Alzheimer disease, under the same conditions as current PBS-listed strengths. Type 2 diabetes mellitus Authority Required (STREAMLINED) listing for triple oral therapy with metformin and a sulfonylurea in patients with type 2 diabetes mellitus. The PBAC recommended the listing of saxagliptin for the treatment of type 2 diabetes mellitus in combination with metformin and sulfonylurea (triple oral therapy). The recommendation was formed on the basis of a cost minimisation analysis compared with dapagliflozin. The equi-effective doses are saxagliptin 5mg and dapagliflozin 10mg. EXELON® Novartis Pharmaceuticals Australia Pty Limited Change to listing (Minor Submission) SAXAGLIPTIN 2.5 mg tablet, 28 5.0 mg tablet, 28 ONGLYZA® AstraZeneca Pty Ltd Change to listing (Major Submission) 17 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION SAXAGLIPTIN and METFORMIN XR saxagliptin 5 mg + metformin hydrochloride 500 mg tablet: modified release, 28 saxagliptin 5 mg + metformin hydrochloride 1000 mg tablet: modified release, 28 saxaglitpin 2.5 mg + metformin hydrochloride 1000 mg tablet: modified release, 56 DRUG TYPE OR USE Type 2 diabetes mellitus LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION Authority Required (STREAMLINED) listing for triple oral therapy with metformin and a sulfonylurea in patients with type 2 diabetes mellitus. PBAC OUTCOME The PBAC recommended that the listing of saxagliptin+metformin XR for the treatment of type 2 diabetes mellitus be extended to include use in combination with a sulfonylurea (triple oral therapy). KOMBIGLYZE® XR AstraZeneca Pty Ltd Change to listing (Minor Submission) 18 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION SITAGLIPTIN SITAGLIPTIN and METFORMIN SITAGLIPTIN and METFORMIN XR sitagliptin 100 mg tablet, 28 sitagliptin 50 mg tablet, 28 sitagliptin 25 mg tablet, 28 sitagliptin 50 mg + metformin hydrochloride 1000 mg tablet, 56 sitagliptin 50 mg + metformin hydrochloride 850 mg tablet, 56 sitagliptin 50 mg + metformin hydrochloride 500 mg tablet, 56 sitagliptin 100 mg + metformin hydrochloride 1000 mg tablet: modified release, 28 sitagliptin 50 mg + metformin hydrochloride 1000 mg tablet: modified release, 56 DRUG TYPE OR USE Type 2 diabetes mellitus (T2DM) – triple oral therapy LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Authority Required (STREAMLINED) listing for triple oral therapy with metformin and a sulfonylurea in patients with T2DM. The PBAC recommended the listing of sitagliptin and the sitagliptin+metformin fixed dose combinations (FDCs) for the treatment of T2DM in combination with metformin and a sulfonylurea (triple oral therapy). The recommendation was formed on the basis of a cost minimisation analysis compared with dapagliflozin in combination with metformin and a sulfonylurea. The equi-effective doses are sitagliptin 100mg and dapagliflozin 10mg. JANUVIA® JANUMET® JANUMET XR® Merck Sharp & Dohme (Australia) Pty Limited Change to listing (Major Submission) 19 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION STRONTIUM RANELATE 2 g granules for oral suspension, 28 x 2g sachets DRUG TYPE OR USE Osteoporosis LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION To not delist the current Authority Required listing for the treatment of severe established osteoporosis. PBAC OUTCOME The PBAC recommended that strontium ranelate should remain listed on the PBS for the treatment of severe established osteoporosis in patients unable to use other osteoporosis medications and without cardiovascular contraindications. PROTOS® Servier Laboratories Change to listing (Major Submission) The PBAC recalled that in April 2014, the TGA issued an alert warning against use of the drug in patients with cardiovascular contraindications and restricting use to patients unable to take other osteoporosis medications. As a result, the PBAC recommended changes to the strontium ranelate restriction in July 2014 and noted that it was of a mind to recommend delisting of strontium from the PBS. However, the PBAC considered that it would be appropriate for the sponsor to have the opportunity to establish the cost effectiveness of strontium ranelate in patients with severe established osteoporosis unable to use other treatments due to contraindication or intolerance. On the basis of meta-analysis presented in the submission, for every 100 patients treated with strontium ranelate in comparison to placebo: Approximately 9 fewer patients would have a new morphometric vertebral fracture (i.e. detectable on x ray or other imaging but not necessarily clinically apparent) over 3 years Approximately 3 fewer patients would have a new clinical vertebral fracture over 3 years Approximately 2 fewer patients would have a new clinical non-vertebral fracture over 3 years There would be no difference in the number of patients with a new hip fracture over 3 years Approximately 5 additional patients would experience a treatment-related adverse event over 3 years The PBAC considered that the claim of superior comparative effectiveness compared with placebo may be reasonable and that the claim of inferior comparative safety compared with placebo was reasonable. A revised base case provided in the pre-PBAC response resulted in an ICER of around $45,000 to $75,000 per QALY. The PBAC considered that if the submission was seeking PBS-listing of strontium ranelate, it would not have considered this ICER to be sufficiently cost-effective for this indication. Accordingly, as the submission is seeking continued listing of strontium 20 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME ranelate, the PBAC considered that a pragmatic approach would be to recommend continued listing of strontium on the condition that the price of strontium is reduced such that the ICER would be around $15,000 to $45,000 per QALY. SUCROFERRIC OXYHYDROXIDE Iron (as sucroferric oxyhydroxice) 500 mg tablet: chewable, 90 VELPHORO® Fresenius Medical Care Pty Ltd Change to listing Hyperphosphataemia To request that the clinical criterion “not to be used in combination with any other phosphate binder” be amended to ensure concomitant use with calcium could occur. Any change would also apply to sevelamer and lanthanum. The PBAC considered that current utilisation of strontium ranelate is relatively small compared with the overall osteoporosis drug market and will continue to decline over time. The PBAC noted the concerns regarding the exclusion of patients taking calcium, aluminium or magnesium based agents concomitantly with sucroferric oxyhydroxide, sevelamer or lanthanum. The PBAC recommended that the clinical criterion for sucroferric oxyhydroxide, sevelamer and lanthanum be amended from “The treatment must not be used in combination with any other phosphate binding agents” to “The treatment must not be used in combination with any other non-calcium phosphate binding agents”. (Other) 21 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION TESTOSTERONE testosterone 1% (50 mg/5 g) gel, 30 x 5 g sachets DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Androgen deficiency Amendment to the revised PBS restrictions to enable access by transgender and intersex patients. The PBAC recommended amending the restriction wording for testosterone to remove the population criterion ‘patient must be male’. The PBAC considered that the remaining requested changes to the restrictions for testosterone products were complex in nature. The PBAC recommended a stakeholder meeting be held between the Department, representatives of the National LGBTI Health Alliance and other consumer representatives, relevant clinicians, the Department of Human Services, the sponsors of testosterone preparations and PBAC members. The aim of this meeting would be to determine an appropriate restriction arrangement (including appropriate prescriber groups) for transgender and intersex populations. A revised restriction would need to reflect the need for these patients to maintain continuity of care with a primary care provider. Chronic obstructive pulmonary disease Authority Required (STREAMLINED) listing for the treatment of patients with chronic obstructive pulmonary disease. The PBAC recommended the listing of tiotropium/olodaterol FDC as an Authority required (STREAMLINED) benefit for the treatment of chronic obstructive pulmonary disease for patients already stabilised on concomitant long acting muscarinic receptor antagonist (LAMA) and long-acting selective β2 agonist (LABA) therapy. testosterone 2% (30 mg/1.5 mL actuation) transdermal solution, 60 actuations testosterone 2.5 mg/24 hours patch, 60 testosterone 5 mg/24 hours patch, 30 Various sponsors National Lesbian, Gay, Bisexual, Transgender, and Intersex (LGBTi) Health Alliance Change to listing (Minor submission) TIOTROPIUM BROMIDE with OLODATEROL HYDROCHLORIDE tiotropium 2.5 microgram/actuation + olodaterol 2.5 microgram/actuation inhalation: solution for, 60 actuations SPIOLTO® RESPIMAT® Boehringer Ingelheim Pty Limited The PBAC recommended the listing on a cost-minimisation basis to the existing LAMA/LABA fixed dose combinations, umeclidinium/vilanterol and glycopyrronium/indacaterol. The equi-effective doses are considered to be tiotropium 5 microgram with olodaterol 5 microgram (two inhalations daily), umeclidinium 62.5 microgram with vilanterol 25 microgram (daily), and glycopyrronium 50 microgram with indacaterol 110 microgram (daily). New listing (Major Submission) 22 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION TRASTUZUMAB solution for subcutaneous injection, 600 mg/5 mL, 1 HERCEPTIN® Roche Products Pty Ltd New listing (Minor Submission) DRUG TYPE OR USE Breast cancer LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Authority Required listing of a subcutaneously administered formulation for the treatment of HER2 positive breast cancer. The PBAC recommended the General Schedule and Schedule 100 Efficient Funding of Chemotherapy (‘Related Pharmaceutical Benefits’) listing of trastuzumab subcutaneous (SC), for patients with locally advanced human epidermal growth factor receptor 2 (HER2) positive breast cancer, early HER2 positive breast cancer and metastatic (Stage IV) HER2-positive breast cancer. The PBAC was satisfied that trastuzumab SC is safe and effective in treatment-naïve patients initiating on this form of trastuzumab; that there is no evidence of significant differences in clinical effectiveness or safety compared with the intravenous (IV) form, and there is no evidence that identifies populations in whom the risks of using the SC form of trastuzumab are disproportionately high. The PBAC was concerned that only very limited clinical efficacy and safety data was provided in support of switching between trastuzumab SC and its IV form, as this is likely to occur in clinical practice. In addition, the chemotherapy partners in practice will be broader than those for which evidence was provided, and trial evidence was not available for the metastatic breast cancer setting. The PBAC concluded that it could not be completely assured of the efficacy and safety of trastuzumab SC in clinical practice. However the PBAC considered that, on balance it was appropriate to recommend trastuzumab SC be listed on the PBS and that patients with breast cancer should be able to access trastuzumab SC via the PBS in the same way as the currently listed trastuzumab IV and without any restriction on switching between the different forms. The recommendation was made on the basis that the listing should, at worst, be cost-neutral for government. 23 JULY 2015 PBAC MEETING – POSITIVE RECOMMENDATIONS DRUG, SPONSOR, TYPE OF SUBMISSION TRASTUZUMAB powder for I.V. infusion, 60 mg vial,1 powder for I.V. infusion, 150 mg vial, 1 DRUG TYPE OR USE LISTING REQUESTED BY SPONSOR / PURPOSE OF SUBMISSION PBAC OUTCOME Gastric cancer Section 100 (Efficient Funding of Chemotherapy) Authority Required listing for the treatment of patients with HER2 positive, metastatic (equivalent to stage IV) adenocarcinoma of the stomach or gastro-oesophageal junction. The PBAC recommended extending the listing of trastuzumab under Section 100 (Efficient Funding of Chemotherapy) for the treatment of HER2 positive, metastatic (equivalent to stage IV) adenocarcinoma of the stomach or gastro-oesophageal junction. This recommendation reflected a wide range of high to very high incremental cost-effectiveness ratios that were acceptable in the context of a modest but potentially meaningful extension of overall survival in a difficult to treat cancer affecting a small number of patients, and with effective controls to limit the financial costs to the PBS. Medicinal food Amendment to the current PBS restriction 'NOTE' to allow an increase in the maximum quantity. The PBAC noted advice from the Nutritional Products Working Party and recommended to amend the current administrative advice note, allowing an increased supply of Peptamen® Junior from the listed maximum quantity of 8 cans up to a maximum of 20 cans to allow a one month supply for an infant or child at an appropriate dose to meet the total nutritional requirements for their respective age range. HERCEPTIN® Roche Products Pty Ltd Change to recommended listing (Major Submission) TRIGLYCERIDES MEDIUM CHAIN FORMULA oral liquid: powder for, 400 g PEPTAMEN JUNIOR® Nestle Health Science (Nestle Australia Ltd) Change to listing (Minor Submission) 24