Protocol - Chart Review - Human Subjects
advertisement

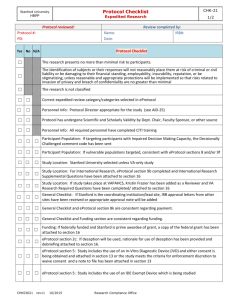
Protocol Checklist - Chart Review Stanford University HRPP CHK-4m 1/1 Medical Research, Clinical Investigations Protocol reviewed: Review completed by: Protocol #: PD: Name: Date: IRB#: If any apply to this study: Specimens ☐ Data Registry ☐ FDA regulated ☐ convert to Expedited and return protocol for additional issues Protocol Checklist check for adequacy or appropriateness Yes No N/A Comment Code or ‘write comment’ ☐ ☐ CITI training ☐ ☐ Protocol Director (their title is appropriate to be a PD) ☐ ☐ ☐ ☐ ☐ ☐ ☐ Study Location: Stanford University should be selected (except for “VA only” studies) write comment ☐ ☐ ☐ General Checklist: Collaborators: Need IRB approval letters from all collaborators IRB APPROVAL ☐ ☐ ☐ General Checklist and Funding sections: Consistent write comment ☐ ☐ ☐ eProtocol 1c: Obtaining Stanford data from STRIDE STRIDE ☐ ☐ ☐ eProtocol 2: Complete (age range in 2b) and consistent with Participant Population Chart Review - age range ☐ ☐ ☐ eProtocol 2c: Rationale for excluding children Chart Review - age range ☐ ☐ ☐ eProtocol 3c: Encryption ☐ ☐ eProtocol 3e: Should be no HIPAA identifiers ☐ ☐ eProtocol 5: Waiver of Consent (all boxes should be “True”) write comment ☐ ☐ eProtocol 5: Waiver of HIPAA Authorization, (all boxes should be “True” and information consistent with eProtocol 3a) write comment ☐ ☐ Yes No ☐ ☐ CITI If PD is a student: Is Academic Sponsor appropriate and APP-9 attached (Review of Scientific and Scholarly Validity, and Oversight (by Academic Sponsor)? Participant Population: At least one choice is marked write comment Academic Sponsor – for student write comment write comment ☐ eProtocol 5: Waiver of Assent if study involves children (all boxes should be “True”) Chart Review - no identifiers write comment Continuing Review Timing – Extended IRB Approval period ☐ Extended approval allowed? “No” if any of the following are indicated: ☐ VA Study ☐ Federal funding (grant or training) (If Stanford is the prime awardee of grant, a copy of the federal grant should be attached in eProtocol 6, and protocol should be consistent with the grant) ☐ Data source is federally funded ☐ Conflict of Interest (eProtocol 4) ☐ Other explanation (e.g., developing software with a company, noncompliance report on a related protocol) CHK02004 rev1 11/14 Research Compliance Office