Neurologic Biomarkers V6 FINAL

Author(s): Berg, Cuoto
Date: January 18, 2014
Question: Do serum levels of neurologic biomarkers NSE, S100 or MBP predict survival or neurologic outcome in children after cardiac arrest?
Settings: University Hospital in-patient
Bibliography (systematic reviews): 1=Topjian, Peds CCM 2009, 2=Fink, Peds CCM 2014, 3=Pfeifer, Resus 2005
Quality assessment Serum Level mcg/L
№ of studies
Study design
Risk of bias
Inconsistency Indirectness Imprecision
Other considerations
Survival or
Good
Neuro
Non-
Survival or
Poor
Neuro
Confidence or Effect p
Relative
Risk
(95% CI) and
Positive
Predictive
Value
Quality Importance
Serum NSE and survival to discharge
2 Pros
Obs
Serious Not serious Not serious Serious Studies 1, 2 Low Important 19.45
15.41
14.11
15.6
25.2
29.8
37.81
56.37
61.23
46.8
75.8
81.5
24 hrs .004
48hrs <.001
72hrs <.001
24hrs .003
48hrs .007
72hrs .004
Serum S100(B) and survival to discharge
2 Pros
Obs
Serious Not serious Not serious Serious Studies 1,2 0.35
0.32
0.33
.021
.029
.024
1.38
1.3
0.66
0.239
0.251
0.167
48hrs .002
72hrs <.001
96hrs .007
24h <.001
48h <.001
72h <.001
Low Important
Serum NSE and neurologic outcome at discharge
2 Pros
Obs
Serious Not serious Not serious Serious Studies 1,2 15.38
11.5
15.1
20.4
17.2
45.8
25.08
36.3
60.8
67
48hr <.001
72hrs <.001
24hrs .019
48hrs .011
72hrs .001
Low Important
Serum S100(B) and neurologic outcome at discharge
1 Pros
Obs
Serious Not serious Not serious Serious Study 2 .023
.034
.028
0.162
0.167
.099
24hr <.001
48hr <.001
72hr <.001
Very
Low
Important
Effect of NSE above cut-off levels 48 hrs after ROSC on poor neurologic outcome or death
1 Pros
Clin
Trial
Not serious
Not serious Serious Very
Serious
Study 3
Effect of S100 above cut-off levels 48 hrs after ROSC on poor neurologic outcome or death
1 Pros
Clin
Trial
Not serious
Not serious Serious Very
Serious
Study 3
>65
>1.5
PPV 97%
RR ~30
(CI ~3-110)
PPV 96%
RR~20
(CI ~2-100)
Very
Low
Very
Low
Important
Important
1 Topjian, Peds CCM, 2009; Study characterized pattern of serum biochemical markers of CNS injury after cardiac arrest. A prospective observational small single-center study of 35 pediatric patients. Some patients had therapeutic hypothermia, not reported separately. This study defined “poor neurologic outcome” as a PCPC change of > or = 2 pre to post-arrest. Lower Neuron Specific Enolase (NSE) at 48 and 72 hours post-arrest predicts survival to discharge and survival with favorable neurologic outcome. Lower S100B at 48 and 72 hours post-arrest predicts survival to discharge. S100B were not associated with neurologic outcome at any timepoint. Note: When excluding subjects with initial PCPC >1 (i.e. subjectes with pre-existing neurologic disease), elevated NSE still predicts death. Elevated S-100B does not predict death at
24 or 48 hrs when subjects with a pre-event PCPC > 1 are excluded.
2 Fink, Peds CCM, 2014 Study characterized pattern of serum biochemical markers of CNS injury after cardiac arrest. A prospective observational small single-center study of 43 pediatric patients. Some patients had therapeutic hypothermia, not reported separately. This study defined “poor neurologic outcome” as a PCPC of 4 to 6 post-arrest. Lower Neuron Specific Enolase (NSE) at 24, 48 and 72 hours post-arrest predicts survival to discharge and survival with favorable neurologic outcome.
Lower S100B at 24, 48 and 72 hours post-arrest predicts survival to discharge and favorable neurologic outcome.
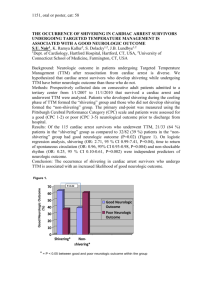
3 Pfeifer, Resus, 2005; Study characterized pattern of serum biochemical markers of CNS injury after cardiac arrest. A prospective clinical trial in a single-center study of
97 adult patients with both non-traumatic IHCA and OHCA. Defines “good or moderate neurologic outcome” as GOS 3-5. The primary finding is that statistical cut-off
levels for both NSE and S-100 at 48 hours after ROSC can predict both death and poor neurologic outcome. Limitations include extrapolation to pediatrics from adult subjects, assayed S100 dimer not the S100B homodimer. Relative risk included in this worksheet is manually estimated from figure 1 in manuscript. Reference group for
NSE and S100 levels were volunteers without neurologic disease, not matched controls. Other limitations: Seizure presence (not controlled in the study group) may effect these levels. Renal failure (not controlled in the study group) effects S100B levels as it is renally excreted.