Experiment 3: Analysis of a Mixture of Carbonate and Bicarbonate
advertisement

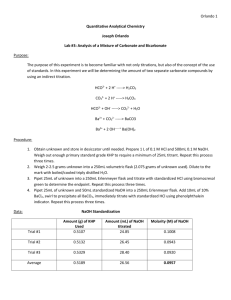
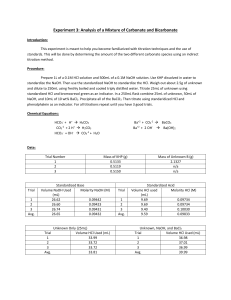
Experiment 3: Analysis of a Mixture of Carbonate and Bicarbonate Andrew Mullenax Objective: The objective of this lab is to determine the amount of two different carbonate species via direct and indirect titration methods. Reactions: 𝐻𝐶𝑂3− + 𝐻 + → 𝐻2 𝐶𝑂3 𝐶𝑂32− + 2𝐻 + → 𝐻2 𝐶𝑂3 𝐻𝐶𝑂3− + 𝑂𝐻 − → 𝐶𝑂3− + 𝐻2 𝑂 𝐵𝑎2+ + 𝐶𝑂32− → 𝐵𝑎𝐶𝑂3 𝐵𝑎2+ + 𝑂𝐻 − → 𝐵𝑎(𝑂𝐻)2 Procedure: Obtain an unknown which contains a mixture of the carbonates of interest and store it in the dessicator Prepare approximately 0.1M HCl and approximately 0.1M NaOH using primary standard grade KHP Accurately weigh enough KHP to require at least 25mL of titrant Use this to standardize your NaOH Repeat until 3 good trials are obtained Repeat using NaOH to standardize your acid Weigh 2.0-2.5g of unknown into a 250mL Erlenmeyer flask. Dilute to the mark with freshly boiled and triply distilled water Pipet a 25.00mL aliquot of unknown into a 250mL Erlenmeyer flask and titrate with standardized HCl using bromocresol green to determine the end point Repeat until 3 good trials are obtained Pipet a 25.00mL aliquot of unknown and a 50.00mL aliquot of standard NaOH into a 250mL Erlenmeyer flask Add 10mL of 10wt% BaCl2, swirl to precipitate all BaCO3 then immediately titrate with standard HCl using phenolphthalein indicator. Repeat until 3 good trials are obtained Data: Volume of 6M HCl used = 16.6mL Mass NaOH used = 2.00g Mass KHP used = 0.51g Bold = Bad trial Standardization of NaOH Trial 1 Mass KHP(g) .5099 Trial 2 .5091 Trial 3 .5098 Trial 4 .5105 Average 0.5098 Volume NaOH added(mL) Concentration of NaOH(M) 27.50 26.74 26.57 26.60 26.85 .0908 .0932 .0940 .0940 0.0937 Standardization of HCl Volume HCl Added(mL) Volume NaOH(mL) Concentration of HCl(M) Trial 1 Trial 2 Trial 3 Average 25.13 25.11 25.13 25.12 25.0 25.0 25.0 25.0 .0932 .0933 .0932 0.0932 Mass Unknown = 2.0368g Titration of Unknown(using bromocresol green) Trial 2 Trial 1 Volume HCl 28.55 29.50 added(mL) Moles of Carbonate .00266 .00275 and Bicarbonate Titration of Unknown(using phenolphthalein) Trial 2 Trial 1 Volume HCl 29.33 29.80 added(mL) Moles of NaOH in .00273 .00278 excess Trial 3 Trial 4 Average 28.60 28.66 28.83 .00267 .00267 0.00269 Trial 3 Trial 4 Average 29.50 29.49 29.53 .00275 .00275 0.00275 Total Moles of NaOH = .004685 Results Moles of Bicarbonate Trial 1 Trial 2 Trial 3 Trial 4 Average Standard Deviation .00191 .00196 .00194 .00194 0.00194 .00002 Moles of Carbonate wt% Bicarbonate wt% Carbonate .00084 .00070 .00073 .00073 0.00075 .00006 5.72 5.87 5.81 5.81 5.8 .06185 2.47 2.06 2.15 2.15 2.21 .18007 Calculations: Moles of KHP: 𝑀𝑎𝑠𝑠 𝐾𝐻𝑃 𝑥 . 5099𝑔 𝑥 1 𝑚𝑜𝑙 204.2212𝑔 1 𝑚𝑜𝑙 = .00250 𝑚𝑜𝑙𝑒𝑠 𝐾𝐻𝑃 204.2212𝑔 Concentration of NaOH: 𝑀𝑜𝑙𝑒𝑠 𝐾𝐻𝑃 1000𝑚𝐿 𝑥 𝑉𝑜𝑙𝑢𝑚𝑒 𝑁𝑎𝑂𝐻 𝑎𝑑𝑑𝑒𝑑 1𝐿 . 00250 𝑚𝑜𝑙𝑒𝑠 1000𝑚𝐿 𝑥 = .0908𝑀 27.50 𝑚𝐿 1𝐿 Concentration of HCl: 𝑣𝑜𝑙𝑢𝑚𝑒 𝑁𝑎𝑂𝐻 𝑥 𝑐𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑁𝑎𝑂𝐻 𝑣𝑜𝑙𝑢𝑚𝑒 𝐻𝐶𝑙 𝑎𝑑𝑑𝑒𝑑 25.0𝑚𝐿 𝑥 .0937𝑀 = .0932𝑀 25.13𝑚𝐿 Moles of Carbonate and Bicarbonate: 𝑉𝑜𝑙𝑢𝑚𝑒 𝐻𝐶𝑙 𝑎𝑑𝑑𝑒𝑑 𝑥 𝑀𝑜𝑙𝑎𝑟𝑖𝑡𝑦 𝑜𝑓 𝐻𝐶𝑙 1000 29.50𝑚𝐿 𝑥 .0932𝑀 = .00275 𝑚𝑜𝑙𝑒𝑠 1000 Moles of excess NaOH: 𝑉𝑜𝑙𝑢𝑚𝑒 𝐻𝐶𝑙 𝑎𝑑𝑑𝑒𝑑 𝑥 29.80𝑚𝐿 𝑥 1𝐿 . 0932𝑚𝑜𝑙 𝑥 1000𝑚𝐿 1𝐿 1𝐿 . 0932𝑚𝑜𝑙 𝑥 = .00278 𝑚𝑜𝑙𝑒𝑠 1000𝑚𝐿 1𝐿 Total Moles of NaOH: 𝑂𝑟𝑖𝑔𝑖𝑛𝑎𝑙 𝑣𝑜𝑙𝑢𝑚𝑒 𝑁𝑎𝑂𝐻 𝑥 𝑀𝑜𝑙𝑎𝑟𝑖𝑡𝑦 𝑁𝑎𝑂𝐻 . 05𝐿 𝑥 .0937𝑀 = .004685 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 Moles of NaOH reacted: 𝑇𝑜𝑡𝑎𝑙 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 − 𝑀𝑜𝑙𝑒𝑠 𝑜𝑓 𝑒𝑥𝑐𝑒𝑠𝑠 𝑁𝑎𝑂𝐻 . 004685 𝑚𝑜𝑙𝑒𝑠 − .00278 𝑚𝑜𝑙𝑒𝑠 = .00191 𝑚𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 Moles Carbonate: 𝑀𝑜𝑙𝑒𝑠 𝐶𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 𝑎𝑛𝑑 𝑏𝑖𝑐𝑎𝑟𝑏𝑜𝑛𝑎𝑡𝑒 − 𝑀𝑜𝑙𝑒𝑠 𝑁𝑎𝑂𝐻 𝑟𝑒𝑎𝑐𝑡𝑒𝑑 . 00275𝑚𝑜𝑙𝑒𝑠 − .00191𝑚𝑜𝑙𝑒𝑠 = .00084𝑚𝑜𝑙𝑒𝑠 𝐶𝑂32− Wt% bicarbonate: 𝑚𝑜𝑙𝑒𝑠 𝐻𝐶𝑂3− 𝑥 61𝑔/𝑚𝑜𝑙 𝑥 . 00191 𝑚𝑜𝑙𝑒𝑠 𝑥 1 𝑥 100 𝑚𝑎𝑠𝑠 𝑢𝑛𝑘𝑛𝑜𝑤𝑛 61𝑔 1 𝑥 𝑥 100 = 5.72% 1 𝑚𝑜𝑙 2.0368𝑔 Wt% carbonate: 𝑚𝑜𝑙𝑒𝑠 𝐶𝑂32− 𝑥 . 00084 𝑚𝑜𝑙𝑒𝑠 𝑥 60𝑔 1 𝑥 𝑥 100 1 𝑚𝑜𝑙 𝑚𝑎𝑠𝑠 𝑢𝑛𝑘𝑛𝑜𝑤𝑛 60𝑔 1 𝑥 𝑥 100 = 2.47% 1 𝑚𝑜𝑙 2.0368𝑔 Conclusions: The purpose of this lab was to determine the relative weight percentage of carbonate and bicarbonate in an unknown sample. The average weight percentages of bicarbonate and carbonate were determined to be 5.80% and 2.21% respectively. Post-lab Questions: A. A primary standard is a pure/stable reagent that can be used directly after weighing. B. A secondary standard is a standard that has been standardized using a primary standard. C. An indirect titration is one in which an excess of standard reagent is added to the analyte and is titrated endpoint. D. The titrant is a reagent of known concentration used to titrate the unknown unknown.