Mechanisms of Arsenite Oxidation under UV
advertisement

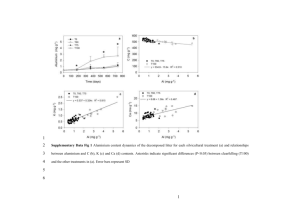
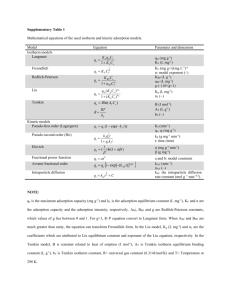
Supplementary Data Solid Surface Photochemistry of montmorillonite: Mechanisms of Arsenite Oxidation under UV-A Irradiation Yanan Yuan a, Yajie Wang a,b, Wei Ding a, Jinjun Li a**, Feng Wu a* a Department of Environmental Science, Hubei Key Lab of Biomass Resource Chemistry and Environmental Biotechnology, School of Resources and Environmental Science, Wuhan University, Wuhan, 430079, P. R. CHINA b School of Chemistry and Environmental Science, Guizhou Minzu University, Guiyang, 550025, P. R CHINA *Corresponding author. Tel: 86-27-68778511, Fax: 86-27-68778511, E-mail: fengwu@whu.edu.cn. **Co-corresponding author. Tel: 86-27-68778511, Fax: 86-27-68778511, E-mail: ljj0410@163.com. 1. Experimental setup 1 Fig. S1. Setup for photooxidation of As(III) on the montmorillonite layer. The temperature and relative moisture of the chamber was maintained at 20 ± 2 °C and 70%, respectively. 2. The emission spectrum of the lamp used for photooxidation Fig. S2. Emission spectrum of the black-light lamp, the main emission wavelength was at 365 nm. 2 3. Dark experiment at different initial As(III) concentration -1 [As(III)]0 (g g ) 100; 50; 200 150; 150 -1 [As(III)] (g g ) 200 100 50 0 0 20 40 80 60 time (h) 100 120 Fig. S3. Effects of initial concentration on As(III) dark reaction. Experimental conditions: pH 6.8, 70% RH. Table S1 The extraction efficiencies of [As]total in controlling experiment. Experiment conditions: [As(III)]0 =100 μg g-1,pH 6.8,70% RH. Time (h) 0 24 48 72 96 120 As(total) photoreaction (μg g-1) 102.19 98.84 99.87 98.16 99.97 95.81 As(total) dark reaction (μg g-1) 97.92 97.48 94.32 99.48 99.47 100.64 4. Dark experiment at different pH values 3 -1 [As(III)] (g g ) 90 80 70 pH = 8.5 pH = 6.8 pH = 5.2 pH = 4.3 60 50 0 20 40 80 60 time (h) 100 120 Fig. S4. Effect of pH value on As(III) dark reaction. Experiment conditions: [As(III)]0 =100.0 μg g-1, 70% RH. 5. Effect of layer thickness in dark Table S2 The relationship between thickness and mass of slurry applied. Mass of slurry applied (g) Thickness (mm) 7.5 0.052±0.001 15 0.104±0.001 22.5 0.162±0.001 30 0.210±0.003 4 -1 [As(III)] (g g ) 95 90 85 80 z = 0.052 mm z = 0.104 mm z = 0.162 mm z = 0.210 mm 75 70 65 0 20 40 60 80 time (h) 100 120 Fig. S5. Effect of thickness on As(III) dark reaction. Experiment conditions: [As(III)]0 =100.0 μg g-1, pH 6.8, 70% RH. 6. Effect of HA in dark experiment 5 HA HA+montorillonite montmorillonite relative intensity 2.0 1.6 1.2 0.8 0.4 0.0 200 300 400 500 600 700 800 wavelength (nm) Fig. S6. Diffuse reflectance spectra of montmorillonite with and without HA. The conditions: 100% montmorillonite, 100% HA, and 5% HA + 95% montmorillonite. -1 [As(III)] (g g ) 90 80 -1 [HA]0 (g g ) 1000 500 250 125 0 70 60 0 20 40 60 80 time (h) 6 100 120 Fig. S7. Effect of HA on As(III) dark reaction. Experiment conditions: [As(III)]0 =100.0 μg g-1, pH 5.2, 70% RH. 7. Effect of additional iron ions in dark experiment -1 [As(III)] (g g ) 90 80 -1 [Fe(III)]0 (g g ) 0 1.0 10.0 100.0 70 60 0 20 40 60 80 100 120 time (h) Fig. S8. Effect of Fe(III) on As(III) dark reaction. Experiment conditions: [As(III)]0 =100.0 μg g-1, pH 5.2, 70% RH. 7 -1 [As(III)] (g g ) 90 80 -1 [Fe(II)]0 (g g ) 70 0 1.0 10.0 100.0 60 0 20 40 60 80 100 120 time (h) Fig. S9. Effect of Fe(II) on As(III) dark reaction. Experiment conditions: [As(III)]0 =100.0 μg g-1, pH 5.2, 70% RH. 8 The influence of different radical scavengers on As(III) adsorption/desorption in dark 8 Csupernatant/Ctotal (%) 100 A: None B: 19.6 mol g-1 CCl4 80 C: 0.1 mol g-1 THB D: 0.5 mol g-1 THB E: 1.0 mol g-1 THB F: 10.0 mol g-1 THB 60 40 20 0 A C B D E F Fig. S10. The influence of different radical scavengers on As(III) desorption in dark. where Csupernatant is the As(III) concentration in supernatant, Ctotal is the total concentration of As(III) in samples. Experimental conditions: [As(III)]0 = 100 μg g−1, pH 4.3, 70 % RH. Cextracted /Ctotal (%) 100 A: None B: 19.6 mol g-1 CCl4 C: 0.1 mol g-1 THB D: 0.5 mol g-1 THB E: 1.0 mol g-1 THB F: 10.0 mol g-1 THB 80 60 40 20 0 A B C D E F Fig. S11. The influence of different radical scavengers on As(III) uptake in dark. where C extracted is the extracted As(III) concentration, Ctotal is the total concentration of As(III) in samples. Experimental 9 conditions: [As(III)]0 = 100 μg g−1, pH 4.3, 70 % RH. 10