Organic3 - Polymers
advertisement

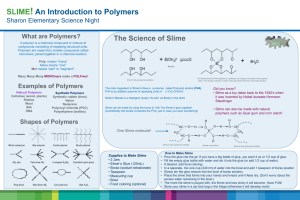
Organic Labs Name: __________________________________ Page 1 of 9 Polymers You can type or handwrite this lab Process Objectives To infer chemical structures from differences in chemical properties. To form a hypothesis about the solubility of STYROFOAM. Learning Objective: After completing this experiment, you should be able to demonstrate and describe the properties of three polymers: one with no cross-linking, one with temporary cross-linking, and one with permanent crosslinking. Introduction Much of our world consists of polymers. The cellulose and lignin of plants, the hair and skin of our bodies, and the starch that we eat are all natural polymers. Polymers are giant molecules consisting of many repeating groups of atoms. These repeating groups, called monomers, form chains that are thousands of atoms long and they often have molecular weights of over 2,000,000 grams. Because these long molecular chains have intermolecular forces of attraction between them, they can be molded into useful objects. Sometimes short bridges of atoms are formed between these long polymeric chains. These bridges can be permanent covalent bonds or temporary H-bonds. The polymer is then said to be “cross-linked:’ Crosslinking gives the polymer new properties. Since the 1930’s, man has made use of these properties in such polymers as nylon, polyester; and polystyrene. Unfortunately, these man-made polymers are not as biodegradable as the natural polymers and have created waste-disposal problems for communities. Land-fill areas, especially near cities, are rapidly becoming filled. Incineration of polymeric waste sometimes produces poisonous gases which pollute our atmosphere. Because of the growing problem of polymer disposal, some communities have banned the use of expanded polystyrene for food containers in fast-food restaurants. The “Polymer Age” has brought many benefits; it has also created many new problems. In this experiment, the polymers polystyrene, polyvinyl alcohol, and sodium polyacrylate will be investigated for some of their properties that have proven so useful to humanity. Polystyrene is one of the most commonly used polymers. Although it is not cross-linked, its strong intermolecular forces of attraction make it useful for constructing products such as radio cases, toys, and lamps. When the polystyrene is expanded (such as in products that are made under the trade name STYROFOAM), it has a very low density and is used in products such as egg cartons, insulation, and fastfood containers. These intermolecular forces of attraction can be decreased by the action of liquid acetone. The attraction of acetone to the chain causes the polymer to lose its shape and become fluid. Organic Labs Name: __________________________________ Page 2 of 9 Polyvinyl alcohol can be weakly cross-linked with the hydrated borate ion. This polymer forms a nonNewtonian gel, which has properties similar to the SLIME that is manufactured by Mattel Toy Corporation. When kept in motion, it forms a semi-rigid mass; when held steady, it flows. Sodium polyacrylate is a strongly cross-linked polymer which has superabsorbent properties. It can form a gel by absorbing as much as 800 times its weight of water. Currently it is used to coat seeds before planting and to remove water from diesel and aviation fuels. Some brands of disposable diapers contain this superabsorbent polymer. For more on Polymers, read Chapter 21, Section 21.3 in the textbook. Take the necessary precautions before beginning this experiment. Wear safety goggles, apron, and gloves. Read all safety cautions in your procedures and discuss them with your teacher. It is important to use good safety techniques while conducting experiments. Show students the dry PVA powder and the 4% Borax solution. Demonstrate the solution’s viscosity by pouring from one beaker to another. Explain how PVA and borax solutions were made. Show the structural formula of polymer chains and borate ion, In water, the borax hydrolyzes to form a borateboric acid buffer system: The B(OH)4 ion is believed to cross-link the polymer chains as shown in Figure 2. Organic Labs Name: __________________________________ Page 3 of 9 When cross-linked materials are stressed, cross-links can be broken and will reform when given time to do so. Each side of the polymer will slide past the other polymer during this process. Organic Labs Name: __________________________________ Page 4 of 9 Exact measurements are NOT necessary. DO NOT waste time measuring exactly. Introduction Solutions of the polymer polyvinyl alcohol (PVA) and polyvinyl acetate can be made into gels by addition of a borax solution. Borax cross-links the polymer chains. In this activity you will investigate interesting properties of gels. “Slime” is a polyvinyl alcohol polymer. “Gluep’ is a polyvinyl acetate polymer. Purpose To observe the effects of cross-linking polyvinyl alcohol and polyvinyl acetate chains with borax. To observe the reversal of the cross-linking process.) Safety 1. Wear protective goggles throughout the laboratory activity. 2. The polyvinyl alcohol and borax solutions are nontoxic, but wash your hands when finished. Keep the gel in plastic bag; keep off clothes and carpets. Procedure Part I. Making “Slime” See questions on the Questions pages. 1, Obtain 10 mL (4 good squeezes of a pipet or 2 teaspoons) PVA solution in a disposable cup. 2. Obtain a wooden stirring stick—a glass stirring rod does not work as well—and stir the PVA solution. Note its viscosity. Is it more or less viscous than water? 3. If desired, add one drop of food color to the PVA. Stir well. 4. Measure 2.5 mL (one good squeeze of a pipet or half a teaspoon) of the borax solution in a 5. Pour the borax solution into the PVA. Stir vigorously until the gelling is complete. 6. Scrape the gel into your hands. Pat and knead it thoroughly to completely mix the components. 7. Test its properties as (density, viscosity, malleability, resistance to pressure). 8. Take the sample home in a zip-closure plastic bag. 9. Thoroughly wash your hands before leaving the laboratory and each time you handle “slime.” Part IA: Making “Gluep” 1. Mix white glue 50:50 with water. Stir well. 2. Measure three or 4 teaspoons glue mixture into a cup. (¼ inch in the bottom of a bathroom cup.) 3. Add color and stir well with a stick. 4. Add two teaspoons (10 mL) borax solution and stir vigorously. (The “gluep” will cling around the stick and can be pulled out, usually in one blob, without much being left in the cup.) 5. Knead the “gluep” well, and observe its properties as before and compare to properties of slime. 6. Store “gluep” in a tightly sealed zip-closure bag. Throw away when it gets moldy. 7. Thoroughly wash your hands before leaving the laboratory and each time you handle “gluep.” Part II – Effect of Acid and Base on “Slime” See questions on the Questions pages. 8. Place a dime-sized piece of slime on a watch glass or Petri dish. Add 2 M HCl dropwise, stirring well with the stick after each drop. When a change is noticed, record your observations. 9. Add 2 M NaOH dropwise to the same sample, stirring well with the stick after each drop. When a change is noticed, record your observations. (Repeat the procedure, if you wish.) 10. Thoroughly wash your hands before leaving the laboratory. Part III Pour 10 mL (4 good squeezes of a pipet or 2 teaspoons of acetone into a small beaker. Put some expanded polystyrene (or a Styrofoam cup) into the acetone. After a few minutes, examine the polystyrene. Using a metal scoop or wooden stick lift out the polystyrene and place it .onto a paper towel. Let it dry for several Organic Labs Name: __________________________________ Page 5 of 9 minutes. Then using an pipet place a few drops of the acetone from the petri dish onto a watch glass and allow to evaporate. Part IV: This part requires exact measurements. Mass a small plastic cup and record the mass. Use the big balance. Put 0.025 grams of sodium polyacrylate into a small plastic cup. Add distilled water until the resulting gel is soupy. Re-mass. Calculate the mass of H2O/gram of sodium polyacrylate. Dispose of the cup and contents in the garbage NOT the sink. Show calculations on the appropriate question on the questions page. Part I. Implications and Applications Read your lab introduction. 4 points each 1. Was the PVA more or less viscous than water? Explain your reasoning. 2. Is covalent or hydrogen bonding responsible for the cross-linking? 3. Why is borax [B(OH)4]- the active ingredient], an effective cross-linker? 4. Draw a representation of the polymer chains: (Be sure to include some water molecules in your drawing; after all, both solutions are 96% water!) 4 pts each a. before cross-linking. b. after cross-linking. Part II. Data Analysis and Concept Development 4 points each 1. How many drops of HCl were necessary to “break the gel?” __________________________ 2. How many drops of NaOH were required to re-gel the polymer? __________________________ 3. Write the equation for the reaction of HCl with NaOH. 4. What happened to the molecule when you added HCl? Organic Labs Name: __________________________________ Page 6 of 9 Part IV: 1. What is the mass of the H2O/gram of sodium polyacrylate that was absorbed? Show Work (6 pts) Questions: 4 points each 1. What evidence suggests that the Styrofoam peanuts did not significantly dissolve in the acetone? 2. The slime that you prepared in Procedure 2 stretches when pulled slowly, but breaks if pulled quickly. Based on its structure, offer an explanation. 3. Expanded polystyrene food containers have many useful properties. They are inexpensive, low in density, and are excellent insulators for both hot and cold foods. Unfortunately, the disposal of these containers presents a land-fill problem. Using what you have learned in this experiment suggest a method for reducing the volume of expanded polystyrene waste. 4. What is the advantage of coating a seed with sodium polyacrylate polymer before planting? 5. What does viscous mean? Give another example of a viscous substance? What makes a substance viscous? 6. Why is PVA (polyvinal alcohol) solutions so viscous? 7. How is a gel like a liquid? solid? 8. Why does acid destroy the properties of the gel? 9. How have the properties changed after addition of NaOH? 10. Why does the base restore the gel’s properties? Organic Labs Name: __________________________________ Page 7 of 9 11. In the lab you did something like the diagram depicts. Explain the diagram and what does it refer to: 12. Write a brief paragraph Slime and Gluep describing the properties of each. What makes them fun? Discuss at least 4 properties. 8 pts 13. What natural material is imitated by rayon? (Site Sources) 6 pts 14. Discuss (one paragraph each) 2 environmental problems with manmade polymers. 6 pts 15. Which chemical in this lab can absorbs up to 800 times its own weight? _________________________________________ 16. Discuss the process of cross-linking in a gel formation. Which experiment s demonstrated this process? 6 pts. Organic Labs Name: __________________________________ Page 8 of 9 Polyurethane Foam System There are many forms of polyurethane such as fibers, coatings, elastomers, flexible foams, and rigid foams. The foam produced in this system is a rigid foam that is used in furniture, packaging, floatation devices, cigarette filters, and many other items. You can produce a rigid polyurethane foam by reacting a difunctional isocyanate with a polyfunctional alcohol, which results in a highly cross-linked polymer system. The reagents involved are specially formulated components Part A an Part B. The side reaction of the dilsocyanate with water in combination with the vaporization of a volatile nonflammable blowing agent provides expanding gases which cause the foam to expand and form a porous structure as the strongly exothermic step polymerization reaction occurs. Part A is a viscous cream colored liquid containing a polyfunctional alcohol, a silicone surfactant, a blowing agent, and a catalyst for the reaction. The blowing agent is trichlorofluoromethane, Freon 11. The two reactions involved are shown below. That involving the reaction of the isocyanate with water produces a carbamic acid which decomposes to yield CO2 gas and an amine. The exothermic nature of the reaction serves to volatilize the blowing agent and. both effects serve to open the polymer network up into a foamed structure. Part B is a dark brown viscous liquid containing a diisocyanate. A general reaction for the polyurethane foam production is: The competing reaction of the dilsocyanate with water is: Materials needed: Polyurethane foam system Parts A and B 2 disposable (bathroom) cups paper towels fume hood, or well-ventilated area wooden stick to stir with acetone goggles, chemical resistant lab apron food coloring Organic Name: ____________________________________Page 9 of 9 Use 2-3 teaspoons of Part A and 2-3 teaspoons of Part B. (about ¼ inch of each in the bathroom cup.) Do not use a graduated cylinder. You may want to put a drop of food coloring into Part A before adding Part B. Stir vigorously until the foam begins to expand and change color slightly. It will expand to 25-30 times its volume and the wood stick may be used to support the foam as it rises, however be sure to remove it while the foam is still soft or plan for it to be a permanent part of the rigid foam formed. Any equal volume mixture may be used, but plan on the correct amount of expansion. To color the foam, add the food coloring to the lighter of the components, Part A, and mix well. The foam dries in 5-10 minutes and should not be handled until then. After that time, any unreacted isocyanate will have reacted with the moisture in the air. Touch the out side of the cup. Questions: 5 pts each 1. What is exothermic mean? 2. Site proof that the reaction is or is not exothermic? 3. In the formulas on the previous page, what does the “R” stand for? What would replace this “R”? 4. Discuss your observations and conclusions. (Cross-linking might be a good concept to include.) 10 pts