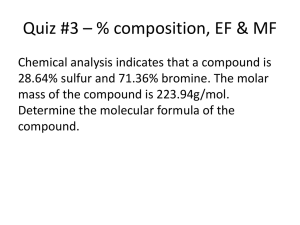
Molecular and Empirical Formulas
advertisement

Molecular and Empirical Formulas Name: ______________________ Period: _______ For each problem, show all work. Include correct units and record answers in correct significant digits. 1. Sulfuric acid, H2SO4, is a strong acid used in many industrial processes. Determine the mass percent of each element in sulfuric acid. 2. Isopropyl alcohol, the main component of rubbing alcohol has the formula C3H7OH. If you wanted to make some isopropyl alcohol, what would be the percent amounts, by mass, that you would need to "construct" this compound? 3. You've just been given a 0.425g sample of a clear, odorless liquid that you were told only contains 7.8% carbon and 92.2% chlorine by mass. How many moles of each element are contained in this sample? Determine the lowest whole number ratio of each element in this compound and use these numbers to write the correct empirical formula for this compound. What is the name of this compound? 5. A compound has the following percentages by mass: barium, 58.84%, sulfur 13.74%, oxygen, 27.43%. Determine the empirical formula for the compound. If this were also the molecular formula for the compound, what would be the name of the compound? 6. Just for kicks, Mr. Tripp decides to heat a 2.542 g sample of aluminum metal in a chlorine gas atmosphere. The mass of the aluminum chloride produced is 12.56 g. Calculate the empirical formula of aluminum chloride. 7. A compound with the empirical formula CH20 was found to have a molar mass between 89 and 91 g. What is the molecular formula of the compound? 8. A compound of smog is found to have 8.4 g of nitrogen and 24.0 g of oxygen. What is its empirical formula? Also provide the name of this compound. 9. Mr. Clauson analyzes a common disinfecting compound composed of hydrogen and oxygen and finds it to have 1.18 g of hydrogen and 18.8 g of oxygen. What is its empirical formula? If this compound has a molar mass of 34 g/mole, what is its molecular formula? ________________ 10. While doing some cleanup in the chemical store room, Ms. Bjorge finds a mysterious bottle containing a compound that she determines has a molar mass of between 165 - 170 g and the following percentage composition by mass: carbon, 42.87%; hydrogen 3.598%; oxygen 28.55%; nitrogen, 25.00%. Determine the empirical and molecular formulas of the compound. 11. Oxalic acid is a compound used in cosmetics and paints. A 0.725 g sample of oxalic acid was found to contain 0.194 g of carbon, 0.016 g of hydrogen and 0.516 g of oxygen. If the molar mass of the acid is 90.04 g/mole, what is the molecular formula? 12. A substance was found at a crime scene. After analysis, 3.7% was hydrogen, 44.4% was carbon and 51.9% was nitrogen. What is the compound? (remember, when percents are given, assume the sample was 100 grams and just change % to grams.) 13. Years ago Roy Plunkett was trying to develop a new refrigerant and stumbled upon a chemical, stuck to the inside of a large container. He analyzed a 15.62 g sample of the polymer material and found it to contain 3.75 g of carbon and 11.87 g of fluorine. What is the empirical formula for this polymer? If the molar mass of this monomer, called Teflon, is 100 g/mole, what is the molecular formula?