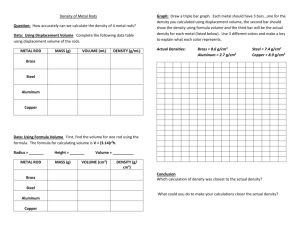
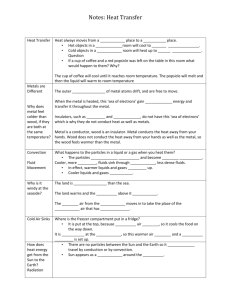
When a new substance is formed with different properties than the
advertisement

Density, Chemical and Physical Changes TEST Total Points: _______/ 100 1. When a new substance is formed with different properties than the original substance it is called a a. Chemical Change b. Physical Change c. Freezing d. Boiling 2. Which is an example of a chemical change? a. Water freezes b. Wood is cut c. Bread is baked d. Wire is bent 3. Metal oxidizing (tarnishing, rusting) is an example of a: a. Chemical property b. Physical property c. Chemical change d. Physical change 4. Which of the following is not a chemical property of matter? a. Flammability b. Corrosive c. Melting point d. Acid/base reactivity 5. When a substance changes appearance, but not into something different. a. Chemical Change b. Physical Change c. Color Change d. Precipitate formation 6. Which of the following is true of chemical properties? a. They describe the phase the substance is in. b. They describe characteristics of a substance such as size, color, and shape. c. They explain how the substance reacts with other substances d. They describe what chemical changes the substance is currently going through. Directions: For questions 7-11, mark each property as chemical (C) or physical (P) A cube of sugar has the following properties: 7. Mass = 2grams 8. Density = 8g/cm3 9. Burns when heated 10. Composed of small, white crystals 11. Bubbles and fizzes when heated Terry and Jean experimented with several items to see what would happen under different conditions. Below is the table they wrote at the conclusion of the experiment. Questions 12-14 Steel Wool Paper Copper Wire Wood splinter Put in water over night Held in candle flame for 30 seconds Rusted Got soppy Got wet Swelled up with water Burned Burned Got hot, bent in middle Burned Connected to the terminals of a 12 volt battery Burned No change Got hot, melted in half No change 12. Which substance changed chemically under allthree threeconditions? conditions? a. steel wool b. paper c. wire d. wood 13. substance changed physically all a. steel wool b. paper c. copper copper d. wood splinter splinter 14. Which What would aisgood conclusion forunder thiswire experiment? a. steel wool the most reactive b. copper paper isbe the most reactive c. wire is the most reactive d. wood splinter is the most reactive 15. What are the four clues to a chemical change? 16. Two clear liquids were mixed together. Which of the observations listed below most likely indicates that a chemical reaction occurred? a. After mixing, the resulting solutions was clear. b. Bubbles formed in the resulting solution. c. Two liquids mixed rapidly d. The temperature of the solution remained constant. Use the Density table below to answer Question #17 You will also need this table for the Case Study in Question 17. A wooden speed boat flipped over and its gasoline leaked into a lake. On your answer sheet in the spaces provided, label how you would expect all of the layers to look. The layers should include: fresh water, sand, the boat, gasoline. Density of Common Substances Material Density (g/cm3) Air 0.0013 Wood 0.61 Gasoline 0.7 Water (ice) 0.9 Water (liquid) 1.0 Aluminum 2.7 Steel 7.8 Silver 10.5 Gold 19.3 A student designed an experiment to test the chemical and physical properties of four known, solid substances (A, B, C, and D) and one unknown solid substance. She suspected the unknown substance was one of the four known substances. The properties she tested are listed in the table. boiling point melting point freezing point mass volume shape reaction to reaction to reaction to water acid heat color odor density 18. The student discovered that the unknown was identical to substance “C” in every property except mass and volume. Could the unknown possibly be identified as “C”? A. yes, if any of properties are the same, then the substances are the same B. yes, mass and volume can vary even in the same substance C. no, mass and volume are important measurements D. no, all properties must match To receive full credit you must show the formula and your work. 19. What is the density of a rock that has a mass of 14g and a volume of 15mL? 20. What is the volume of a tank that can hold 18,754Kg of methanol whose density is .788g/cm3 21. How many Kg of mercury would fill a 5L container if the density of mercury is 13.6g/cm3 22. What is the density of a board whose dimensions are 5.54cm x10.6cm x 199cm and whose mass is 28.6Kg? 23. A sample of lead is found to have a mass of 32.6g. A graduated cylinder contains 2.8mL of water. After the lead sample is added to the cylinder the water level reads 5.7mL. Calculate the density of the lead sample. C Case Study 24. Use the information in the data table to answer the following questions • BE SURE TO ANSWER AND LABEL ALL PARTS OF THIS QUESTION. • Show all your work (diagrams, tables, or computations) on your Answer Sheet. • Explain in writing how you did the work in order to receive full credit. Metal A Metal B Metal C Properties Silvery white, nonmagnetic, ductile, malleable Mass 500 g Shiny yellow, soft, nonmagnetic, metallic, malleable 137 g Soft, white, lustrous, most ductile and malleable, good conductor of heat 75 g Volume 185 ml 7.1 cm3 7.1 ml Melting point 660.37 °C 1064.43 °C 962 ºC Someone in the lab did not put the metals back properly! The scientist now has three unlabeled samples of pure metals and he needs to determine the identity of each metal. Using your knowledge of physical and chemical properties, help the scientist identify these 3 metals. A. Identify which ONE of the following properties is the best option to help the scientist determine the identity of the pure metal in each sample: color, melting point, mass, or volume. B. Explain why/how the property you identified in part (A) can be used to determine the identity of the pure metal in each sample. The scientist cuts each of the metal samples into two smaller pieces. C. Is the property that was used in part A to determine the identity of the metal affected when each sample is cut into two pieces? Explain your answer. D. Name 2 other tests the scientist might use to clearly determine the identity of the 3 samples. The scientist can also use density to determine the identity of the pure metal in each sample. E. How can the scientist determine the density of the pure metal in each sample? F. Calculate the density of the 3 samples using the information given in the data table. G. Identify the 3 samples by comparing your answers in (F) to the information in the Density Data Table from Question 19. The scientist then adds one of the small pieces of metal A to a clear solution. It starts to fizz, a white solid appears and an odor is produced. H. Was this a physical or chemical change? Explain your answer.