History of atomic structure wkst.
advertisement

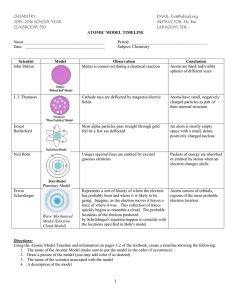
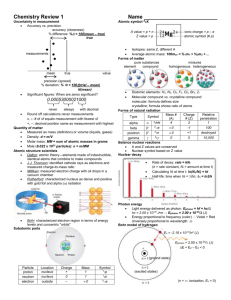
Chem I HISTORY OF ATOMIC STRUCTURE REVIEW Name___________________ Period______ For the following, place the appropriate letter for the scientist that matches the following incomplete statements. The letters in the list that follows can be used more than once. A. Rutherford B. Boyle C. Dalton D. Newton E. Democritus F. JJ Thomson G. Millikan _____1. Stated that atoms of the same element are identical and that atoms of different elements are different. _____2. Scientist that determined that there must be a central, dense, positive center called a nucleus. _____3. Used the Gold Foil Experiment to help support his findings. _____4. Determined that atoms are composed of a negatively charged particle called an electron. _____5. Stated that matter cannot be created or destroyed only chemically rearranged. _____6. One of the first to write down their thoughts about atomic structure in the 17th century. For the following, write the appropriate information on the line provided. 7. Through the use of a cathode ray tube, __________________ was able to calculate the ratio of an electron’s charge to its mass. 8. Although his views were not supported, __________________ in 400 B.C. proposed that the world was made from empty space and tiny particles called atoms. 9. The scientist that proposed the plum pudding model of atomic structure in which the negative electrons were scattered around randomly occurring positive charges was _________________. 10. List the 4 aspects of Dalton’s Atomic Theory: 11. _______________was the first scientist to accurately calculate the charge of an electron and the mass of an electron. 12. List the 4 main results of Rutherford’s Gold Foil Experiment: 13. The two scientists who are responsible for our knowledge of the electron being the negatively charged subatomic particle are __________________ and __________________. 14. List one of the two possible scientists: __________________ stated his belief in atomic structure during the 17th century but he did not have quantitative data to support his belief. 15. This scientist, __________________, offered the first logical quantitative explanation of atomic structure. 16. The scientist that predicted the presence of neutrons in the nucleus was __________________.